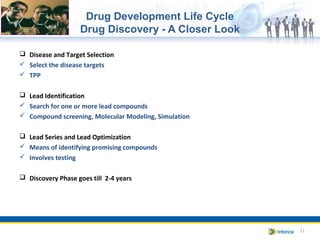
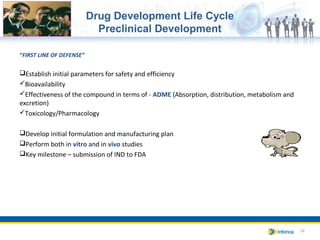
The document provides an overview of the pharmaceutical and biotech industries, focusing on drug definitions, classifications, and the drug development life cycle. It outlines the various phases of drug development, including discovery, preclinical studies, and clinical trials, while emphasizing the role of regulatory bodies in ensuring product safety and efficacy. Additionally, it highlights different healthcare products and their importance in patient treatment across various care settings.