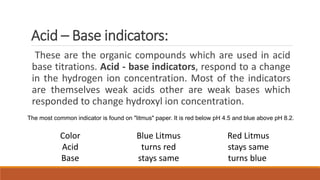
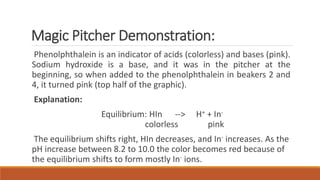
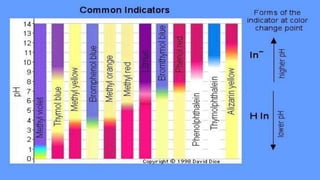
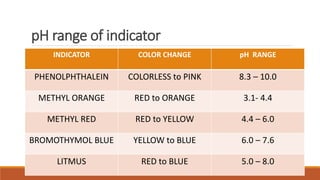
pH indicators are organic compounds that change color based on the pH of a solution. They are used to check pH and indicate the endpoint of reactions. Common indicators include litmus, which is red below pH 4.5 and blue above pH 8.2, and phenolphthalein, which is colorless below pH 8.2 and pink above pH 10. In a demonstration, phenolphthalein was added to solutions containing sodium hydroxide, turning them pink, but when one solution was then mixed with acetic acid, the indicator turned colorless due to the drop in pH caused by neutralization.