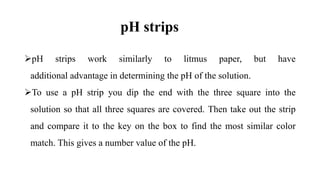
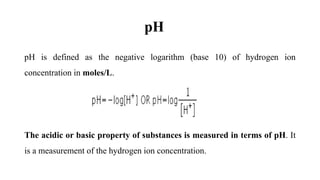
The document discusses hydrogen ion concentration, defining it as critical for determining the acidic or basic nature of solutions via pH, which is the negative logarithm of hydrogen ion concentration. It also covers the pH scale, indicators for testing acidity and alkalinity, and the importance of buffers in maintaining stable pH levels in solutions. The text explains various indicators such as litmus paper, pH strips, universal indicators, and phenolphthalein in testing the acidity or alkalinity of solutions.

![Hydrogen ion concentration
Hydrogen ion concentration is the composition of hydrogen ions in a
solution.
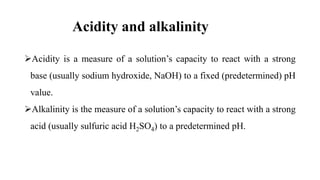
The acidic, basic and neutral character of a solution can be found
out by the concentration of hydrogen ions [H+].
The large bracket sign, [ ], indicates molar concentration (mol/L).](https://image.slidesharecdn.com/lec1-180206104903/85/Lecture-1-PH-and-Litmus-paper-2-320.jpg)


![The product of [H+] and [OH-] always remains constant even if the value
for one of the species changes.
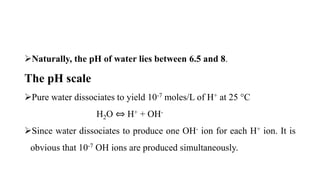
[H+] x [OH-] = 10-14
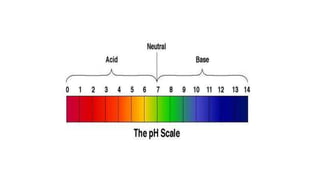
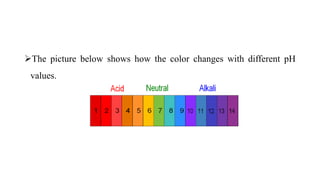
The pH scale is usually represented as ranging from 0 to 14, with pH 7 at
25 °C representing neutrality.
Acid conditions increase as pH values decrease and alkaline conditions
increase as pH values increase.](https://image.slidesharecdn.com/lec1-180206104903/85/Lecture-1-PH-and-Litmus-paper-7-320.jpg)