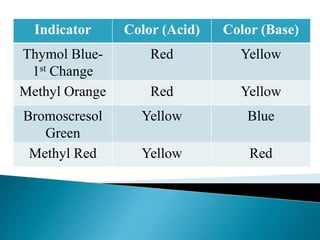
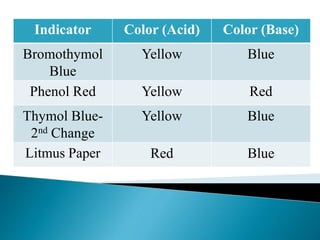
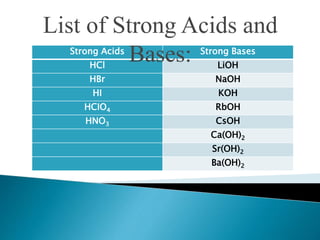
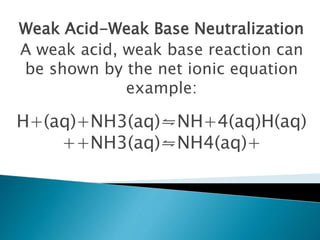
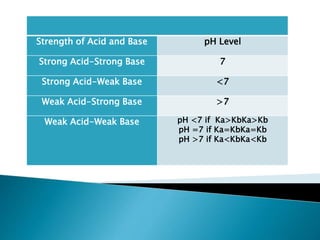
Acid-base indicators change color based on pH, acting as weak acids or bases. Common indicators and their colors in acidic versus basic conditions are listed. Neutralization occurs when an acid and base react to form water and a salt, involving the combination of H+ and OH- ions. The pH of solutions formed from strong acid-strong base neutralization will be 7, while strong acid-weak base solutions will be less than 7 and strong base-weak acid will be greater than 7.