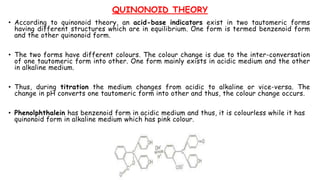
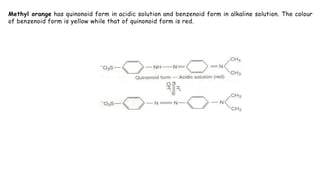
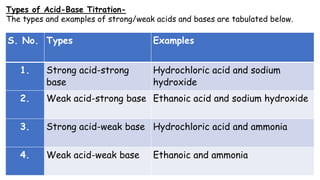
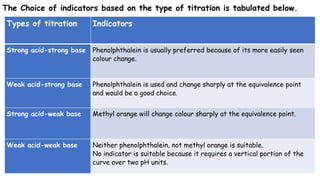
This document discusses acids, bases, and acid-base titration. It defines acids as substances that can donate protons and bases as substances that can accept protons. It explains that acids are sour and turn litmus red, while bases are bitter and slippery and turn litmus blue. Acid-base indicators are then discussed, including how they change color with pH. Two theories of acid-base indicators are described: Ostwald's theory involving ionization equilibrium, and the quinonoid theory involving tautomeric forms. Finally, different types of acid-base titrations are listed along with the typical indicators used for each type.





![Phenolphthalein
It is represented as HPh. This indicator being a weak acid ionises in solution to a small extent as
follows:
HPh H+ + Ph-
Colourless Pink
Applying law of mass action, we get
K = [H+][Ph-]/[HPh]](https://image.slidesharecdn.com/acid-basetitration-210616021235/85/Acid-base-titration-6-320.jpg)

![Methyl orange-
It is a very weak base and can be represented as MeOH. It is ionized in solution to give Me+ and OH-
ions.
MeOH Me+ + OH-
Yellow Red
Applying law of mass action,
K = [Me+] [OH-]/[MeOH]](https://image.slidesharecdn.com/acid-basetitration-210616021235/85/Acid-base-titration-8-320.jpg)