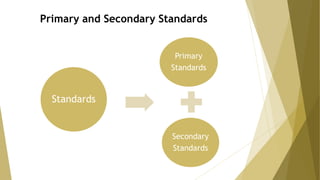
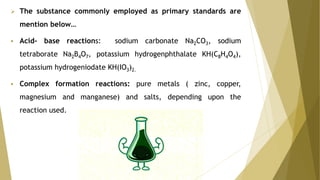
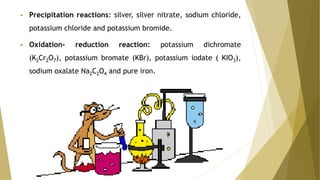
Primary standards are very pure substances used to determine unknown concentrations, typically through titration. They are stable, anhydrous, and have a high molecular weight. Common primary standards include sodium carbonate, potassium hydrogenphthalate, and pure metals.
Secondary standards are standardized against primary standards for use in specific analyses. They have less purity and stability than primary standards but their solutions remain stable for long periods. Secondary standards are used to calibrate analytical methods and include substances titrated against a primary standard.