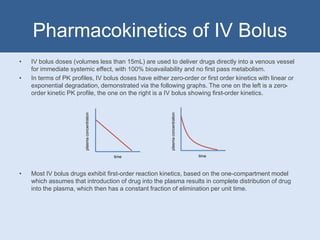
This document summarizes different parenteral routes of drug administration including intravenous, intramuscular, and subcutaneous. It discusses key aspects of each route such as typical volumes administered, advantages, and disadvantages. It also covers pharmacokinetic concepts for intravenous bolus doses such as zero-order and first-order kinetics, and equations for calculating parameters like volume of distribution, half-life, loading dose, and time to steady-state.













![Pharmacokinetics - Equations
• The steady-state concentration (Css) is calculated by D/Cltotal x τ, where τ is the dosing interval.
• The loading dose (LD) is calculated by Vd x Css (peak).
• A drug has a Vd of 35L and a t1/2 of 6 hours. Toxicity is not seen at levels below 30mg/L and the
initial dose (D) is 1000mg. Repeated administration requires concentrations of 5 – 10mg/L.
• The Cltotal can be calculated by: (0.693 x 35)/6 = 4.0425
• The amount of time (in hours) after which a second dose should be given is calculated by: 5 =
C0e-λt.
• The λ can be calculated by: 4.0425/35 = 0.1155
• The C0 is calculated by: 1000/35
• Therefore t (5mg) = ln[5/(1000/35)]/0.1155 = 15.09064333 hours
• Therefore t (10mg) = ln[10/(1000/35)]/0.1155 = 9.089369043 hours
• The Css can be calculated by: 1000/(4.0425 x 12 hours) = 20.61439633mg/L
• The patient should not receive a loading dose in this case, as the C0 is 28.571428mg/L, which is
near the upper limit of 30mg/L above which toxicity occurs.](https://image.slidesharecdn.com/parenterals-160120210405/85/Parenterals-15-320.jpg)
