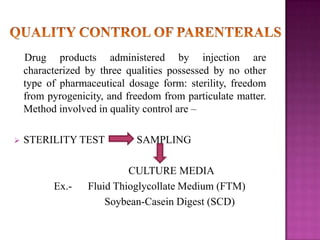
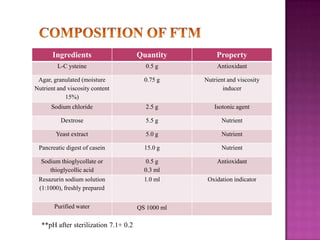
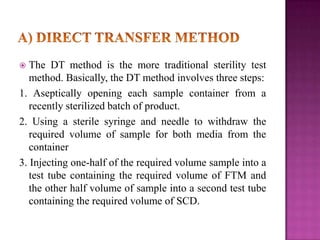
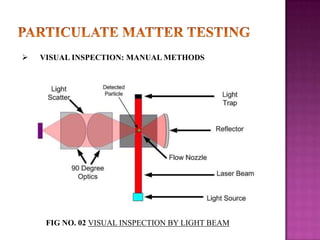
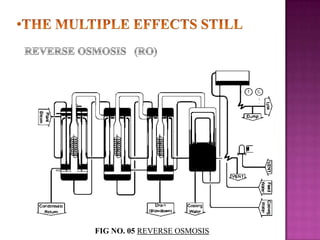
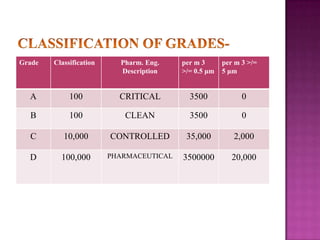
The document discusses sterile products and parenteral dosage forms. It defines sterile products as dosage forms that are free of viable microorganisms, including parenteral, ophthalmic, and irrigating preparations. Parenteral products must be free of microbial contamination and toxic components as they are injected directly into the body. The document lists different types of sterile products and routes of parenteral administration. It emphasizes that sterile products must meet strict quality standards including being sterile, free of pyrogens, and particulate matter.