The document discusses factors to consider when designing dosage forms for medicines, including the active drug ingredient and excipients added. Excipients are carefully selected to modify the physical and chemical properties of the dosage form to achieve the best possible medicine.
Dosage forms should provide a uniform, repeatable, and safe dose of the drug to achieve a therapeutic effect for patients. However, patient variation in genetics, metabolism, and illness can impact response. Excipients are used to help achieve consistent responses across populations.
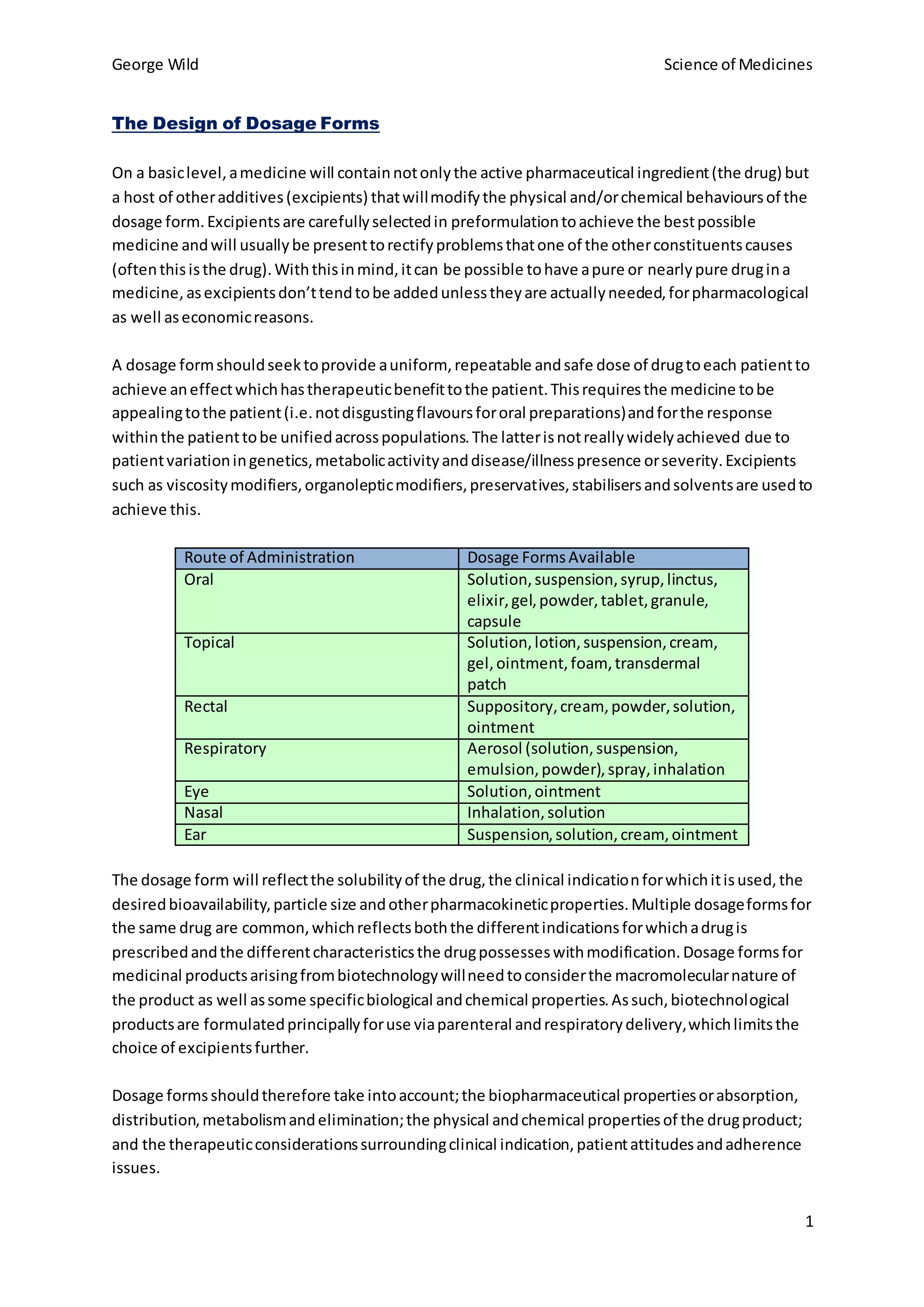
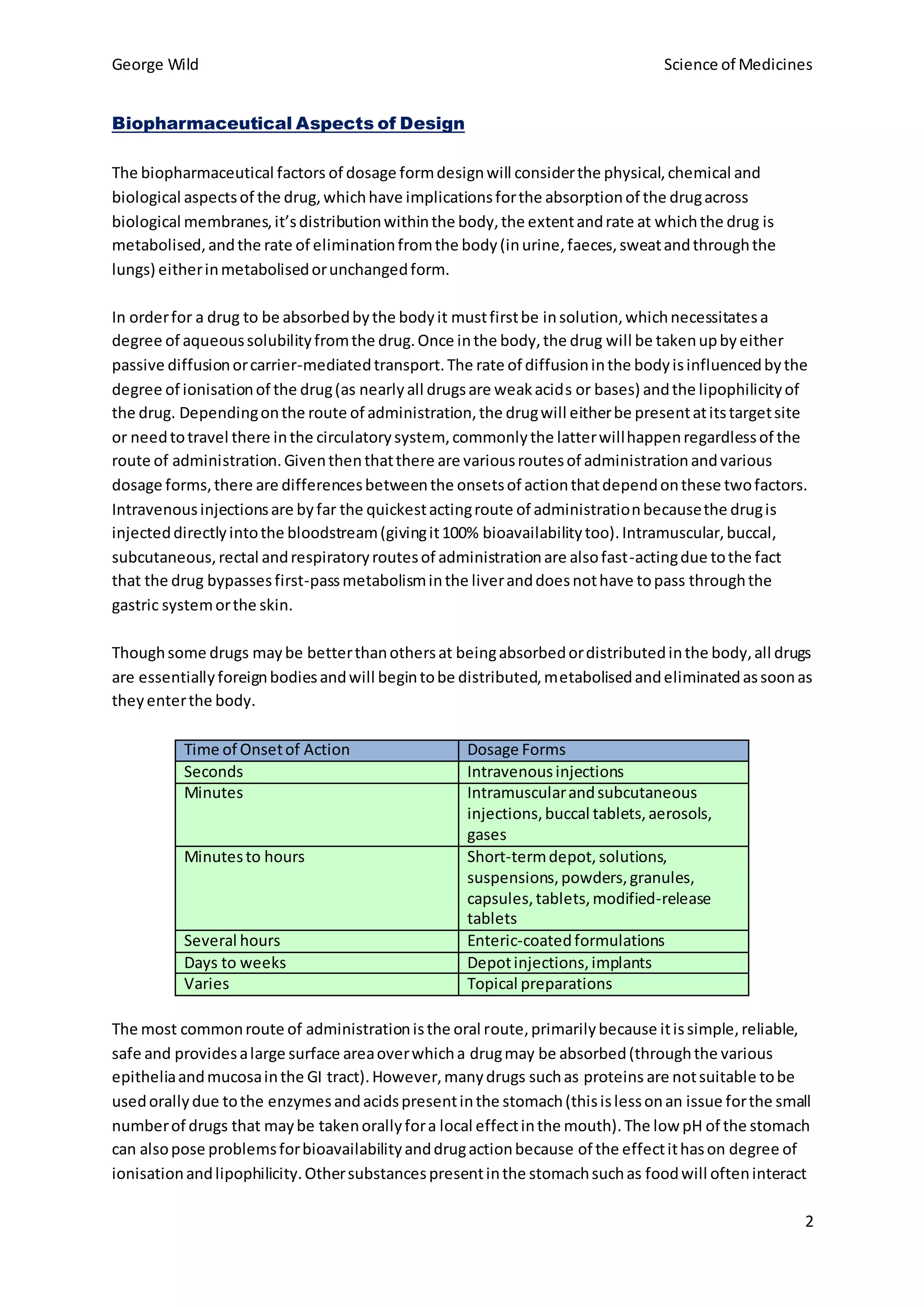
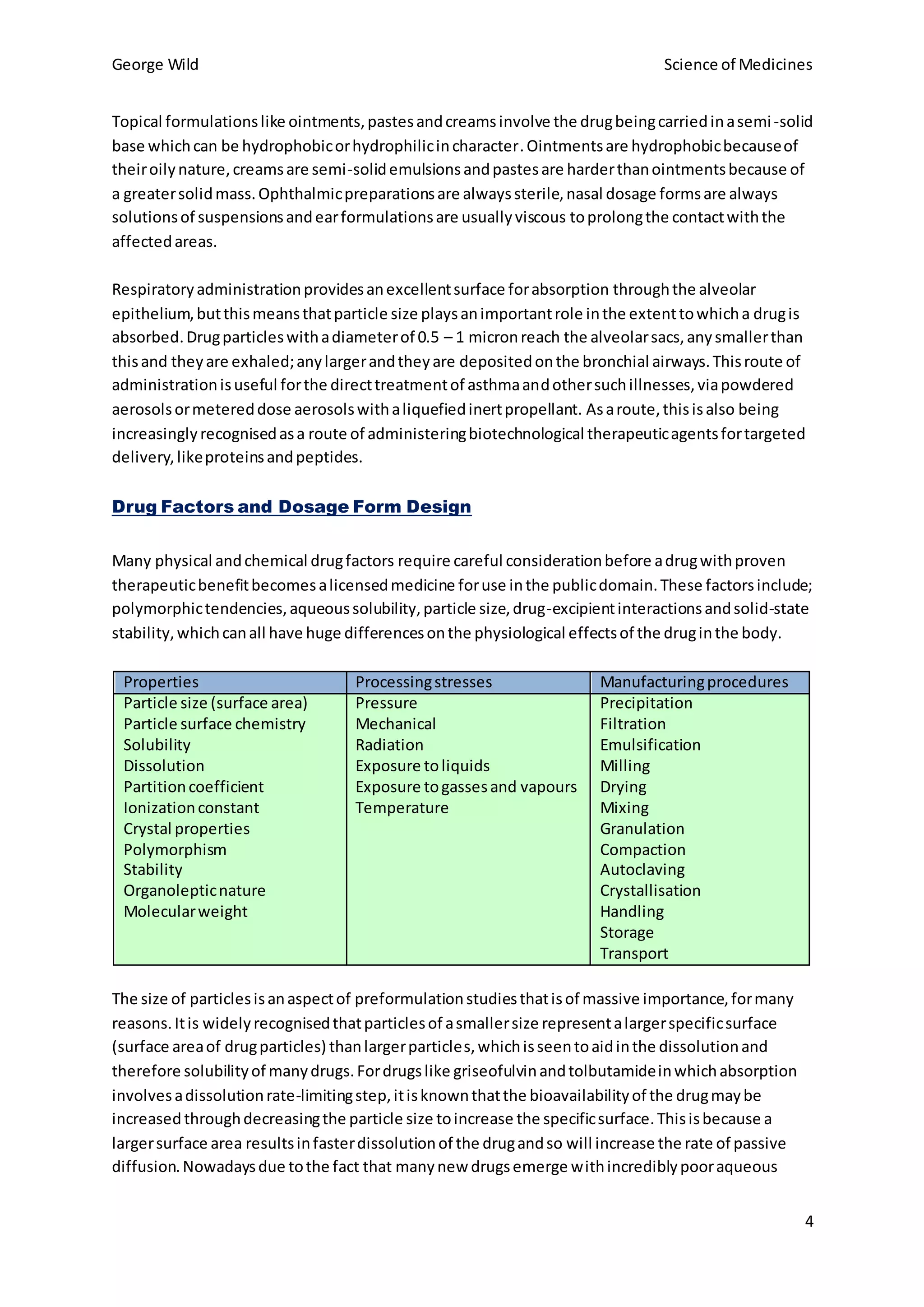
The choice of dosage form reflects properties of the drug like solubility, intended use, and desired pharmacokinetics. Multiple dosage forms for a single drug