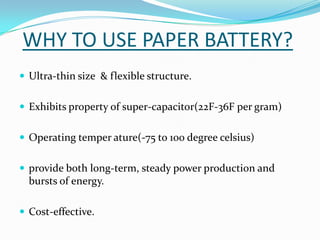
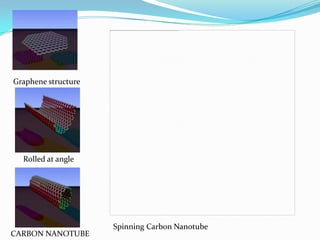
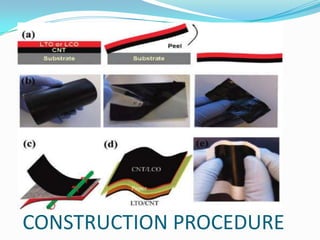
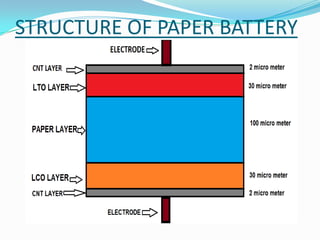
The document discusses paper batteries, which are flexible, ultra-thin energy storage devices made by combining carbon nanotubes with paper. A paper battery acts as both a battery and supercapacitor. It has advantages over traditional lithium-ion batteries such as being thinner, more flexible, and operating over a wider temperature range. Paper batteries are constructed by coating carbon nanotube films onto substrates and sandwiching them between electrolyte layers and paper. They work by producing electrons through the interaction of electrolytes during charging and discharging. Potential applications include powering small electronics and medical devices.