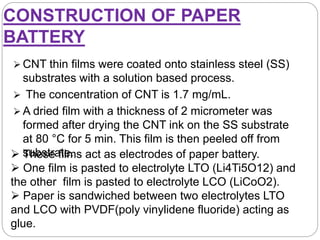
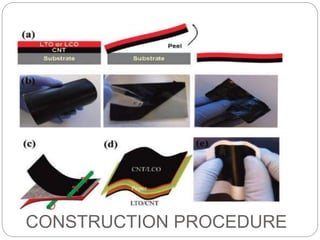
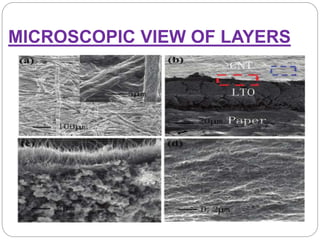
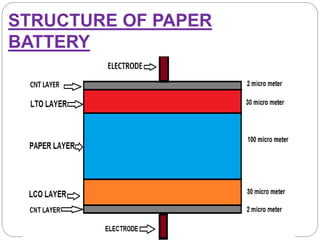
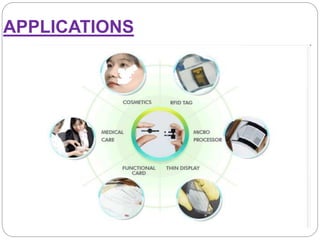
The document summarizes a paper battery, which is an ultra-thin, flexible energy storage device made by combining carbon nanotubes with paper. A paper battery functions as both a battery and supercapacitor. It has advantages over traditional lithium-ion batteries such as being thinner, more flexible, and operating over a wider temperature range. The document describes the components and construction of the paper battery, including how carbon nanotubes are used as electrodes and different materials are layered and bonded with paper to store and release energy. Potential applications include powering small electronics or medical devices.