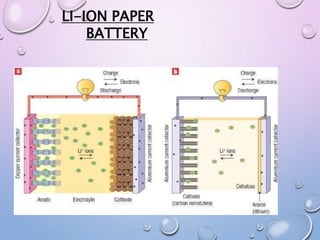
The document discusses paper batteries, which are flexible energy storage devices made by combining carbon nanotubes with paper. Paper batteries were developed in 2007 as an alternative to conventional batteries, which have limitations like limited life, weight, leakage, and environmental hazards. A paper battery works similarly to lithium-ion batteries but incorporates all components into a lightweight paper-based sheet. It has advantages like flexibility, low cost, safety, and can use body fluids as electrolytes. However, scaling up paper batteries and the cost of nanotubes remain challenges to commercialization. Potential applications include powering electronics, medical devices, and electric vehicles.