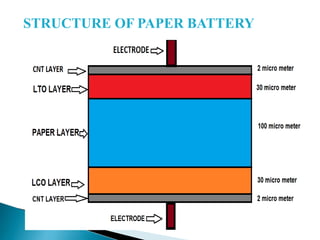
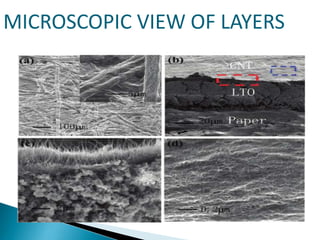
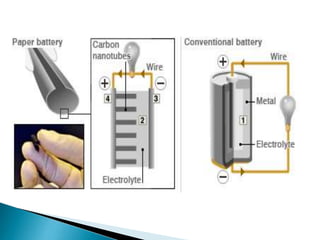
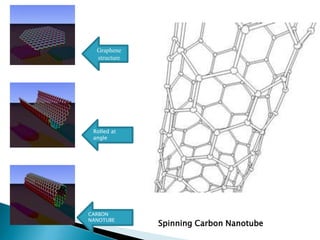
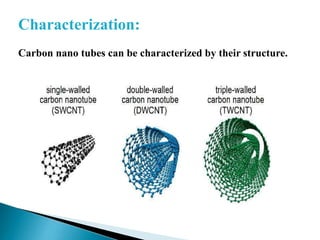
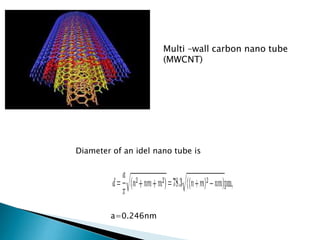
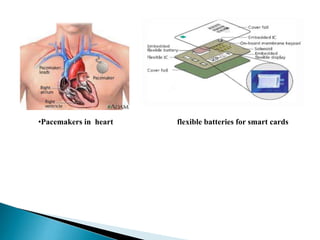
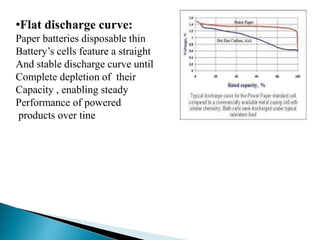
This document discusses paper batteries as a flexible, ultra-thin energy storage device made by combining carbon nanotubes with paper. Paper batteries act as both batteries and supercapacitors. The document then compares paper batteries to conventional lithium-ion batteries. It provides details on the construction and working of paper batteries, including how they are made by applying carbon ink to paper and connecting electrodes. Paper batteries are described as a potentially cost-effective alternative power source for applications like wearable devices due to their flexible thin design.