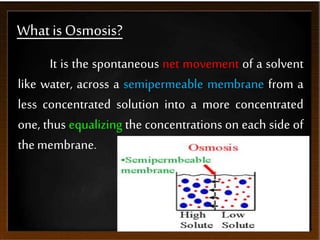
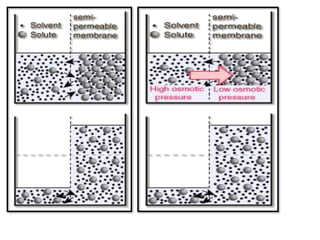
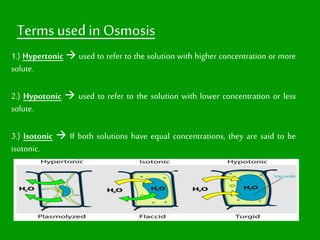
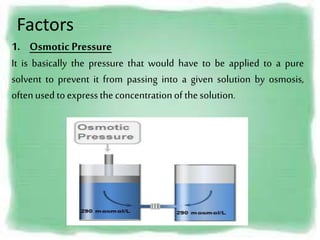
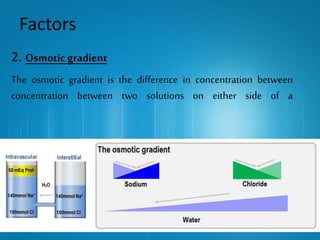
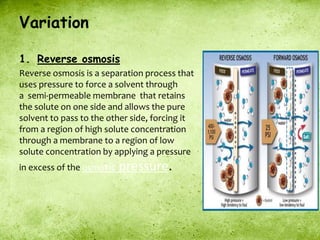
This presentation introduces the process of osmosis. It defines osmosis as the spontaneous movement of water across a semi-permeable membrane from a less concentrated solution to a more concentrated one. It distinguishes osmosis from diffusion, which does not require a membrane. The presentation outlines key terms like hypertonic, hypotonic and isotonic solutions. It provides examples of osmosis in applications like plant water uptake, food preservation, and kidney dialysis. In conclusion, osmosis is the diffusion of water molecules through a selectively permeable membrane to equalize concentrations.