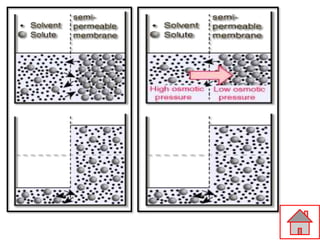
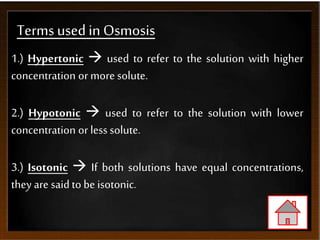
The document discusses the process of osmosis and related concepts. It defines osmosis as the spontaneous movement of water across a semi-permeable membrane from a less concentrated solution to a more concentrated one. It distinguishes osmosis from diffusion and defines terms like hypertonic, hypotonic, and isotonic solutions. Examples of osmosis applications include how it assists plants and is used in food preservation and kidney dialysis. It also discusses osmotic pressure and provides examples of calculating osmotic pressure and solute concentration.