1. Quantum mechanics describes the behavior of matter and light at the atomic scale, which is very different from classical mechanics. Particles have both wave-like and particle-like properties.
2. The de Broglie hypothesis proposed that all particles have an associated wavelength that depends on their momentum. This was confirmed experimentally by observing electron diffraction patterns.
3. Heisenberg's uncertainty principle states that it is impossible to precisely measure both a particle's position and momentum simultaneously. This limits our ability to predict the future behavior of particles.































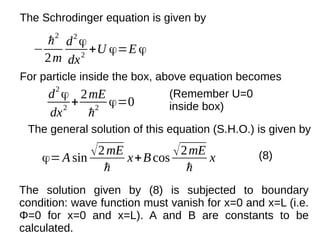





![ϕn=A sin
nπ x
L
n=1,2,3 ……
Φn
is a well behave wave function because :
1. For each value of n, Φn
is finite, single valued function
of x and Φn
and ∂Φn
/∂x are continuous.
2. The integral |Φn
|2
over all space is finite.
∫−∞
∞
|ϕn|2
dx=∫
0
L
|ϕn|2
dx=A2
∫
0
L
sin2
(
nπ x
L
)dx
=
A2
2
∫
0
L
(1−cos(
2nπ x
L
))dx
=
A2
2
[∫
0
L
dx−∫
0
L
cos(
2nπ x
L
)dx]](https://image.slidesharecdn.com/quantum-170517170829/85/Quantum-38-320.jpg)
![=
A2
2
[∫
0
L
dx−∫
0
L
cos(
2nπ x
L
)dx]
=
A
2
2
[x−
L
2nπ
sin(
2n π x
L
)]
0
L
=
A
2
2
[L−
L
2nπ
sin(
2n π L
L
)−0−
L
2nπ
sin(
2nπ 0
L
)]
=A
2
(
L
2
)
⇒∫−∞
∞
|ϕn|
2
dx=A
2
(
L
2
)i.e.
But if Φ is to be normalized that means A should be
assigned a value such that equation (12) should be equal to
1.
(12)](https://image.slidesharecdn.com/quantum-170517170829/85/Quantum-39-320.jpg)









