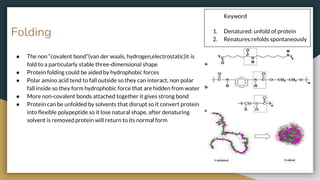
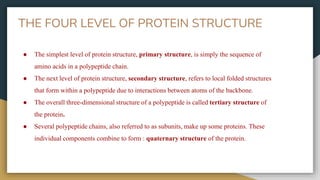
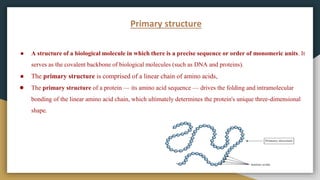
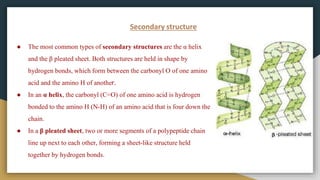
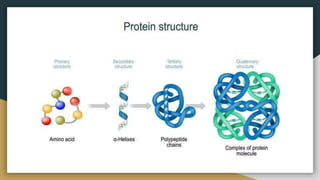
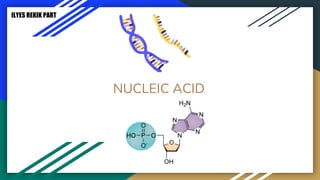
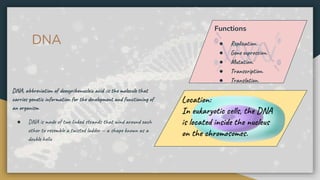
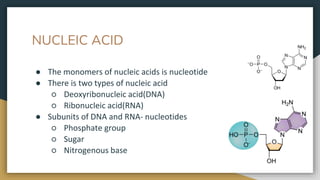
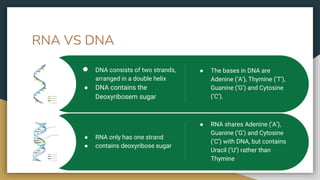
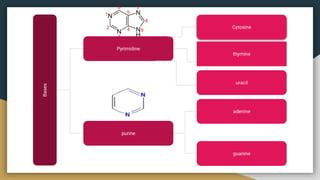
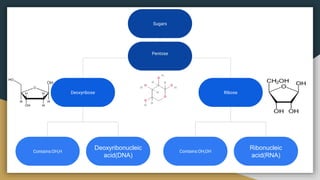
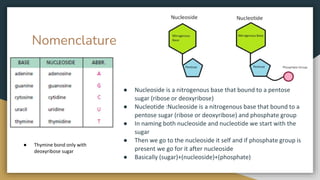
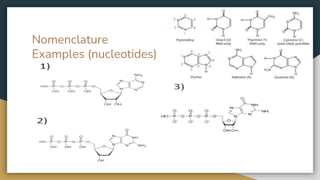
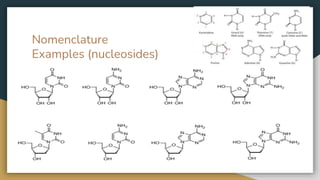
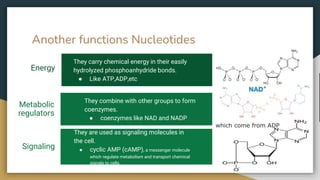
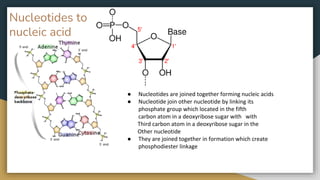
Protein, RNA and DNA are made up of smaller building blocks. Protein is made from amino acids that are linked through peptide bonds. RNA and DNA are made from nucleotides that contain a phosphate group, a sugar (ribose in RNA and deoxyribose in DNA), and a nitrogenous base. There are four levels of protein structure - primary, secondary, tertiary and quaternary. RNA and DNA store and transmit genetic information through their structures and functions such as replication, transcription and translation.