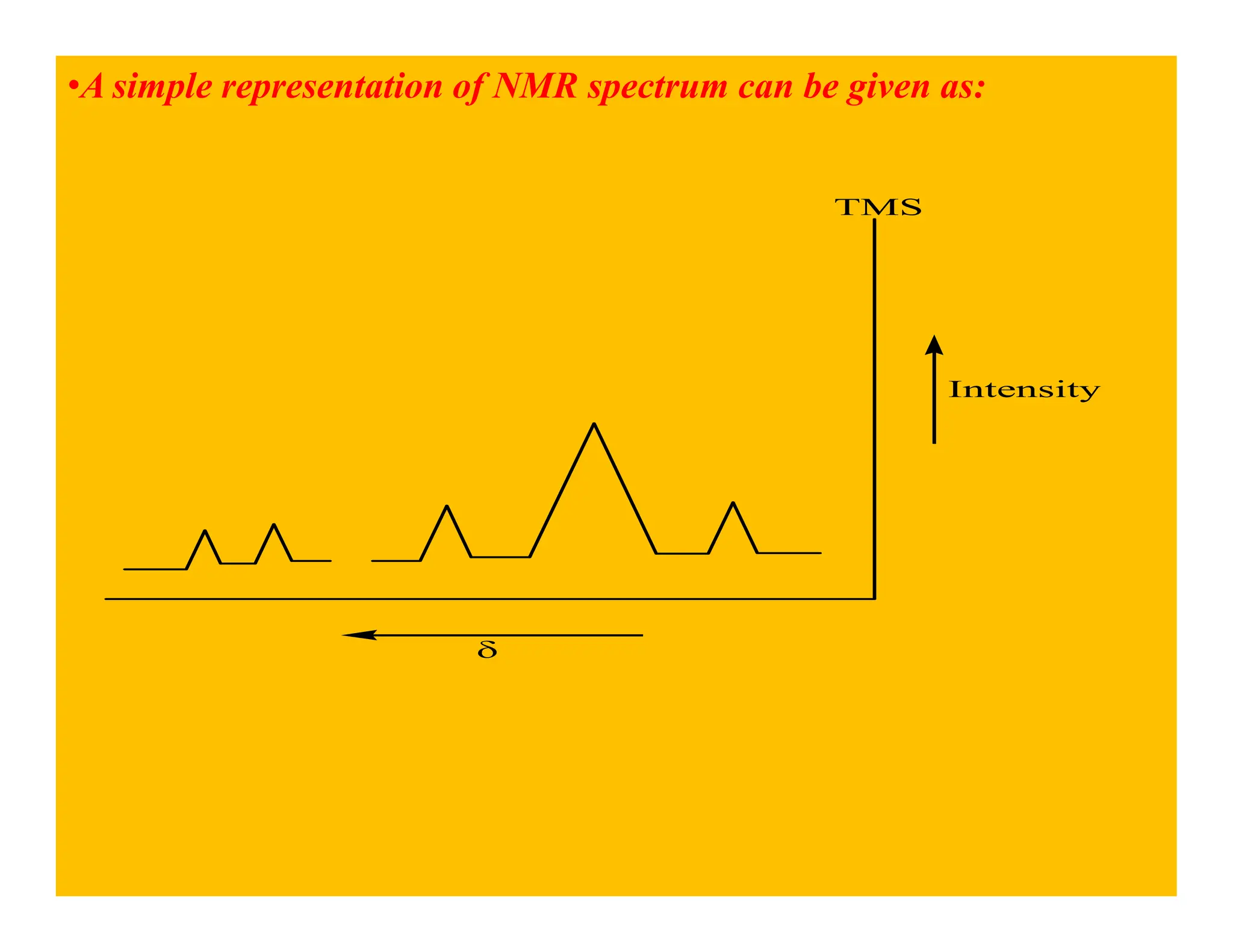
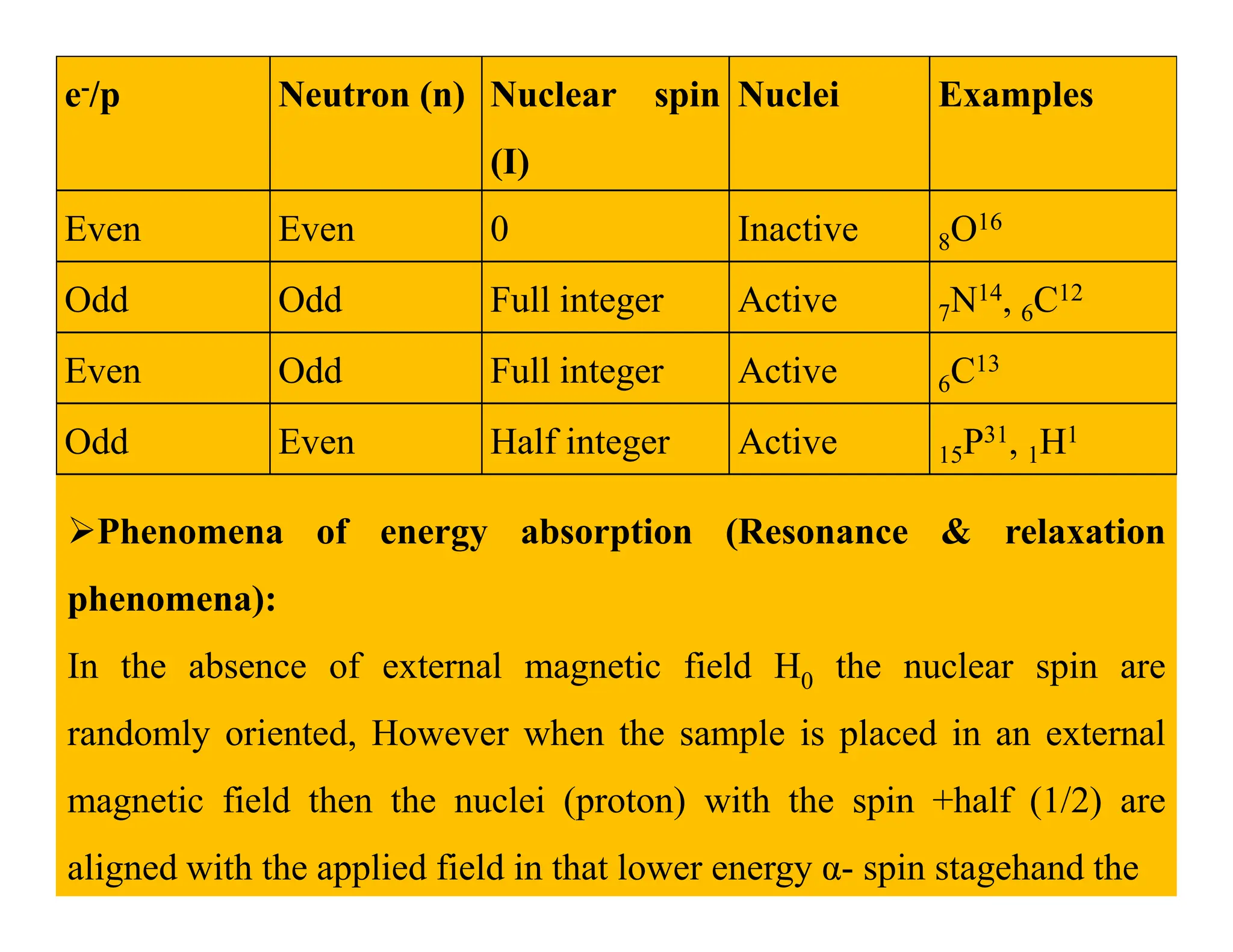
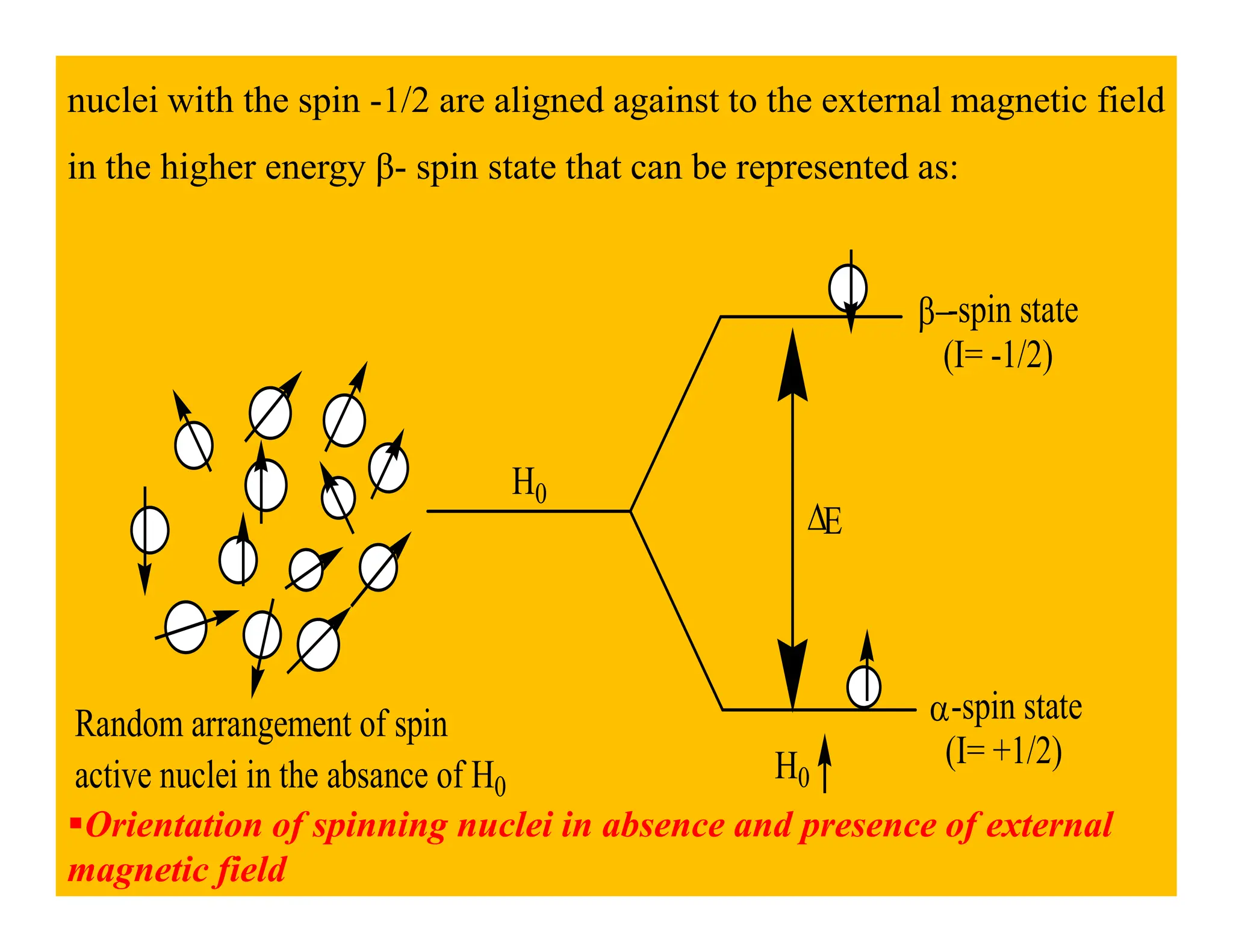
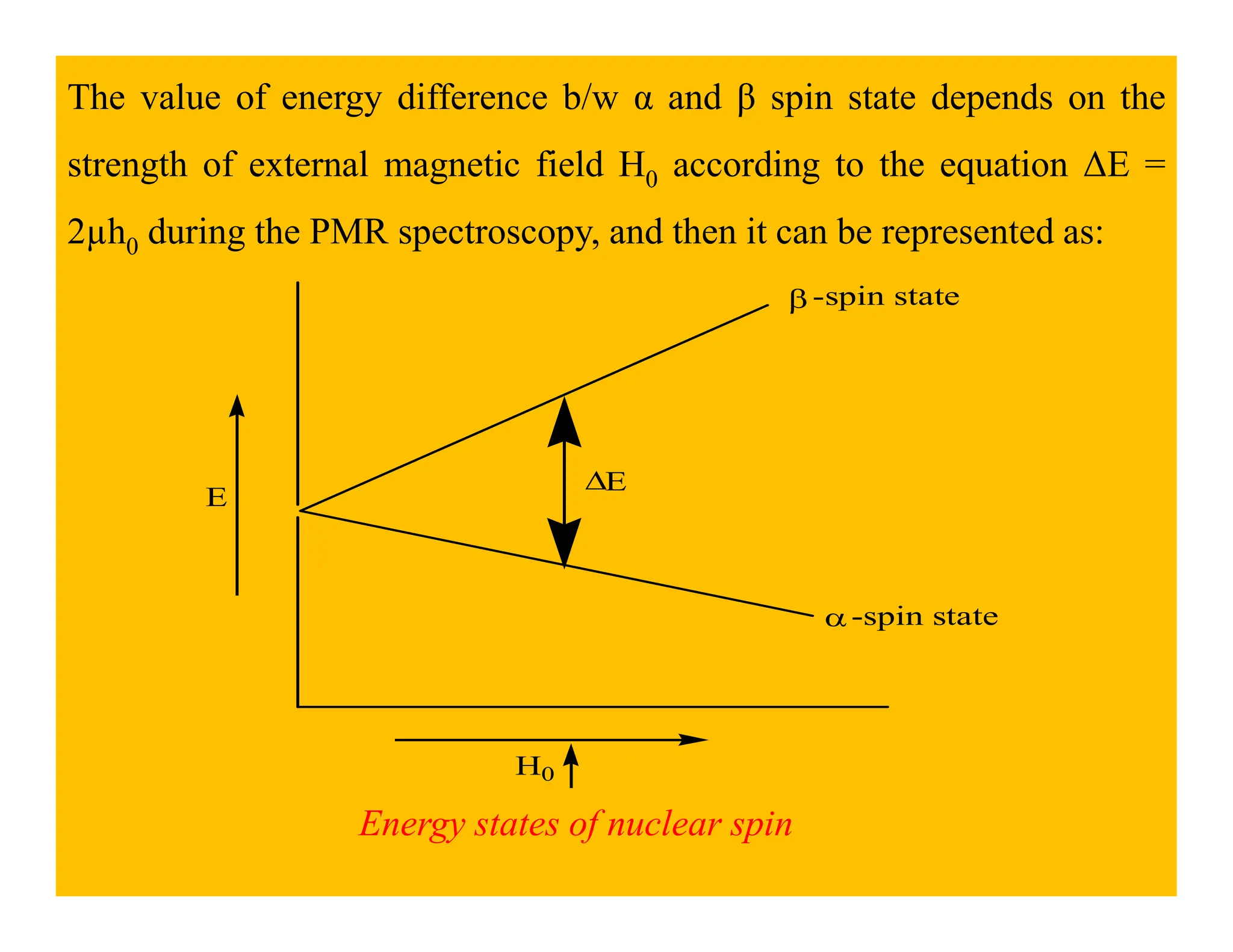
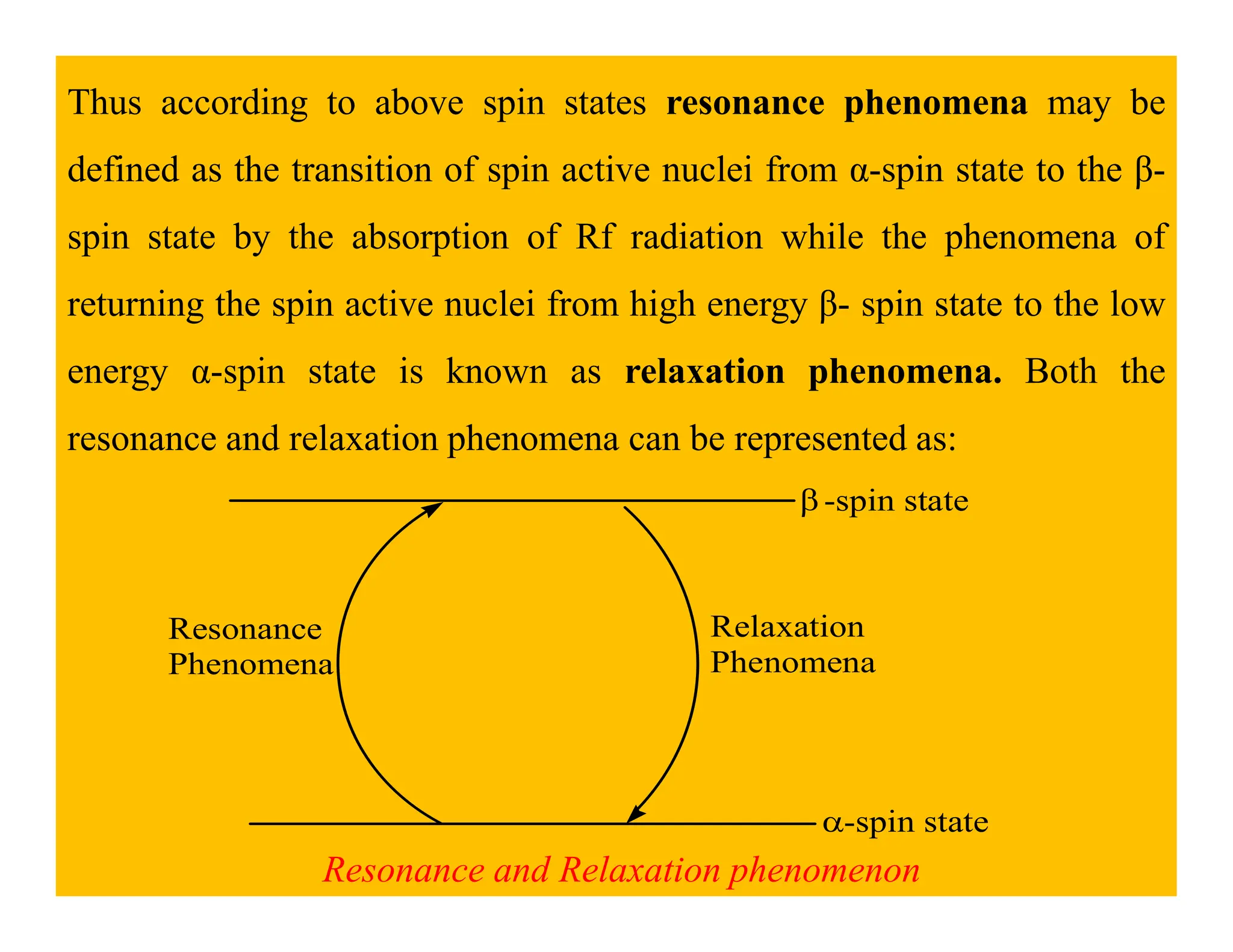
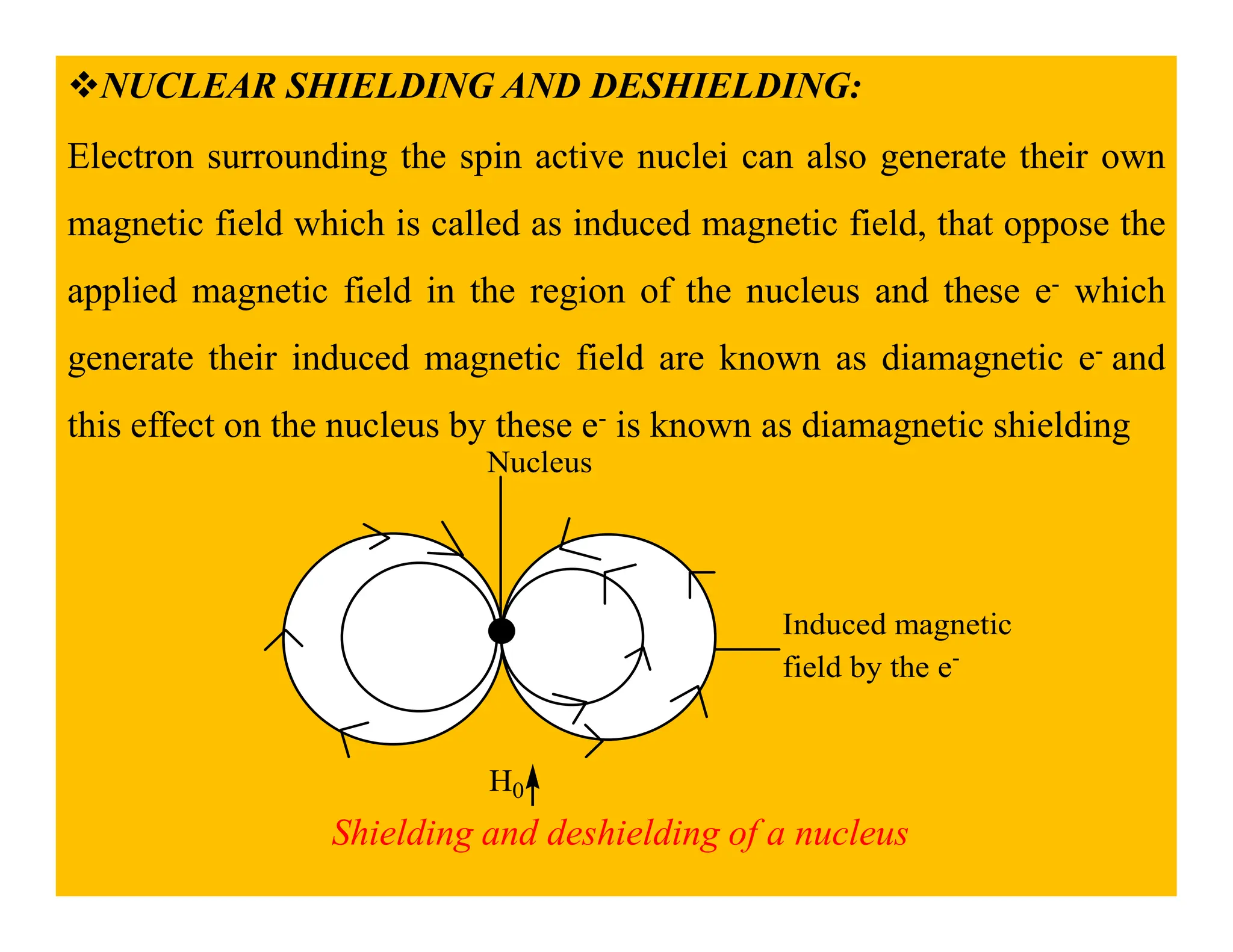
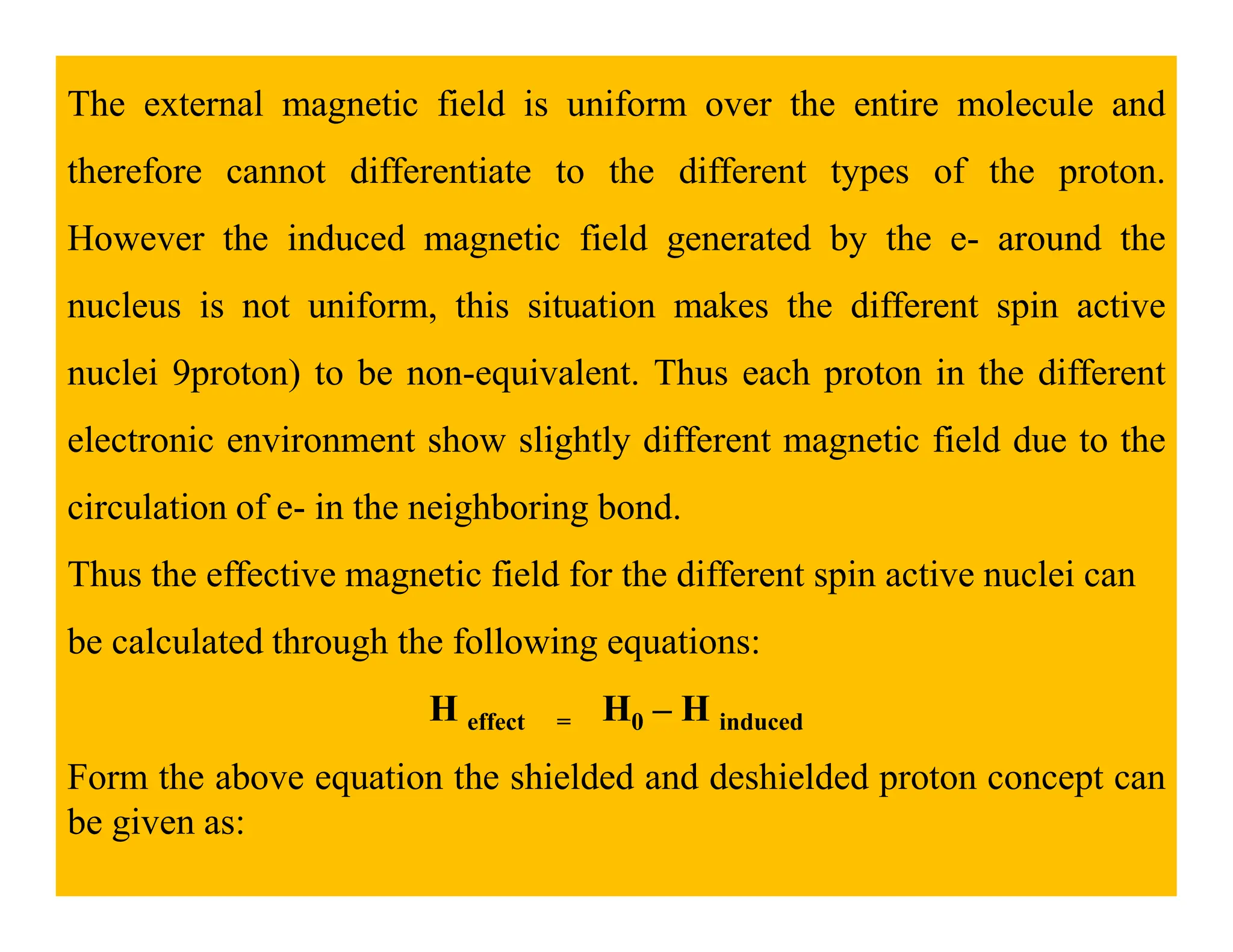
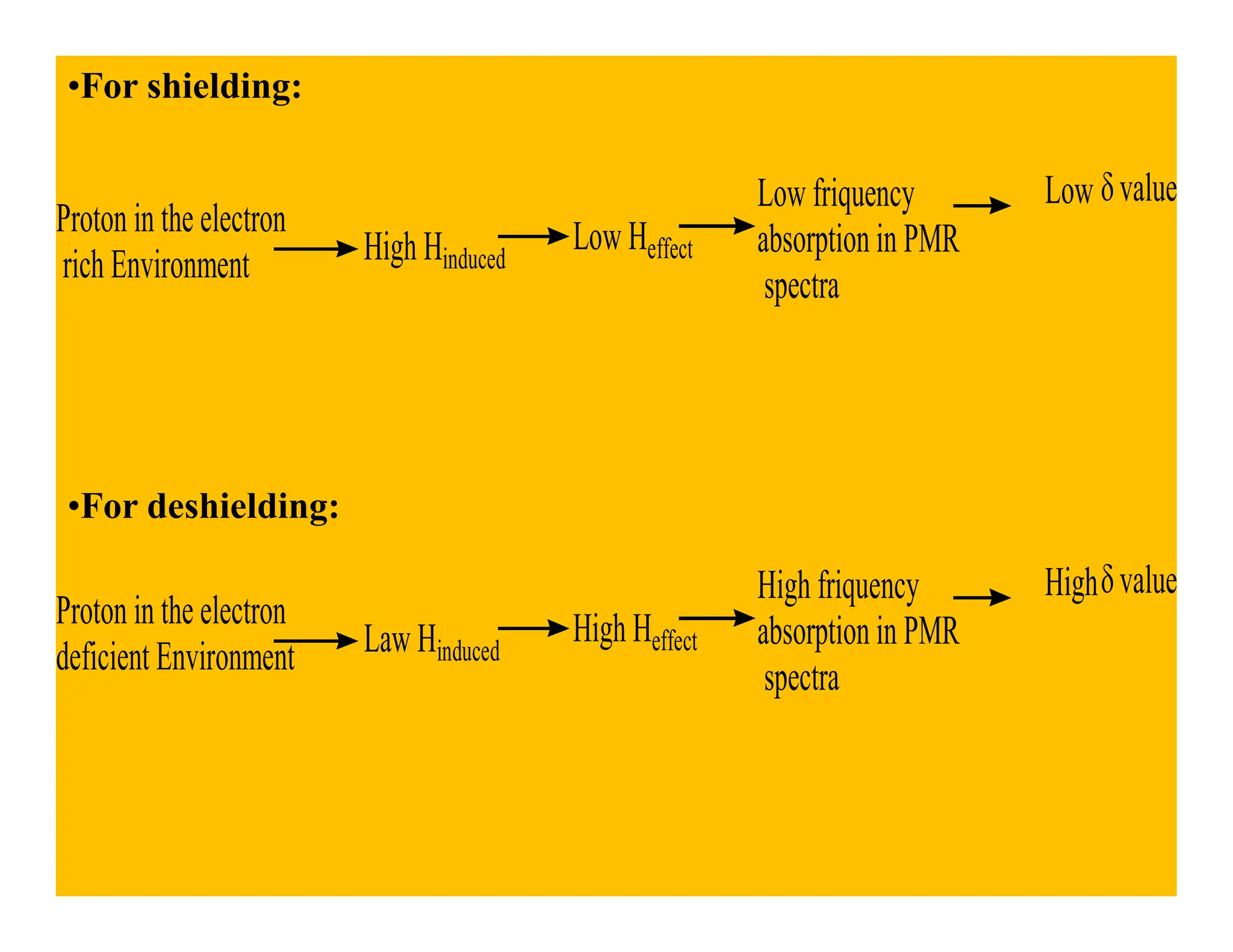
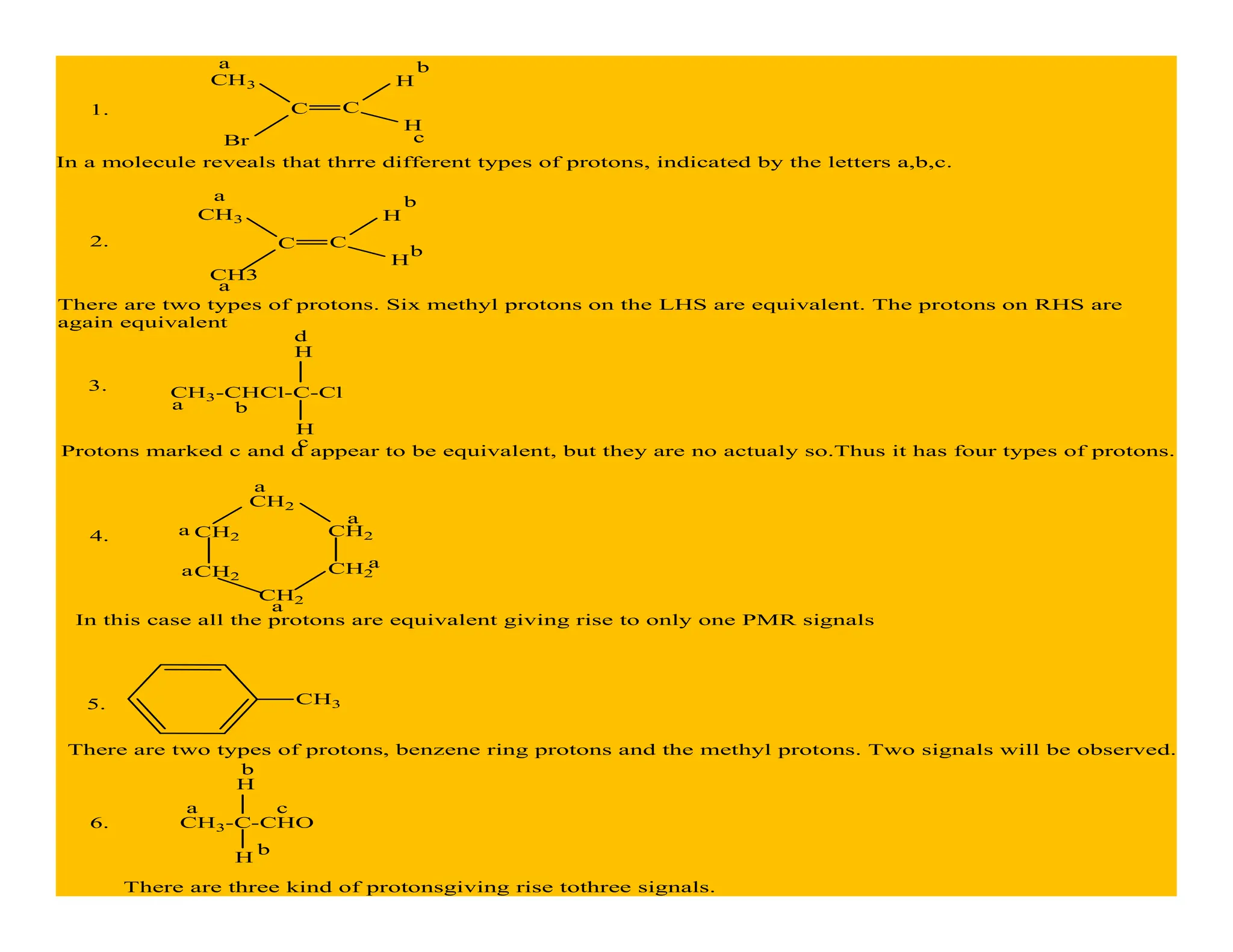
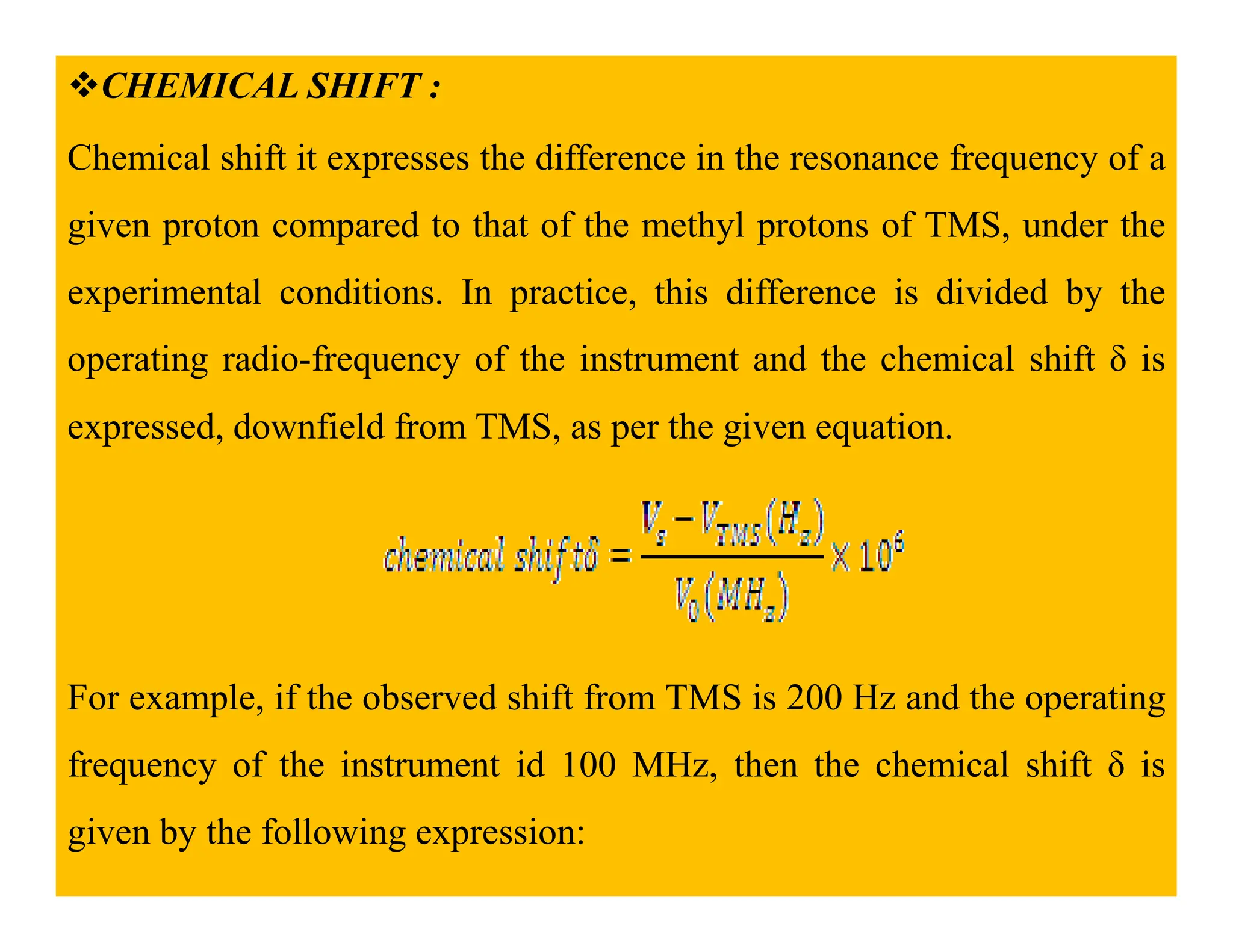
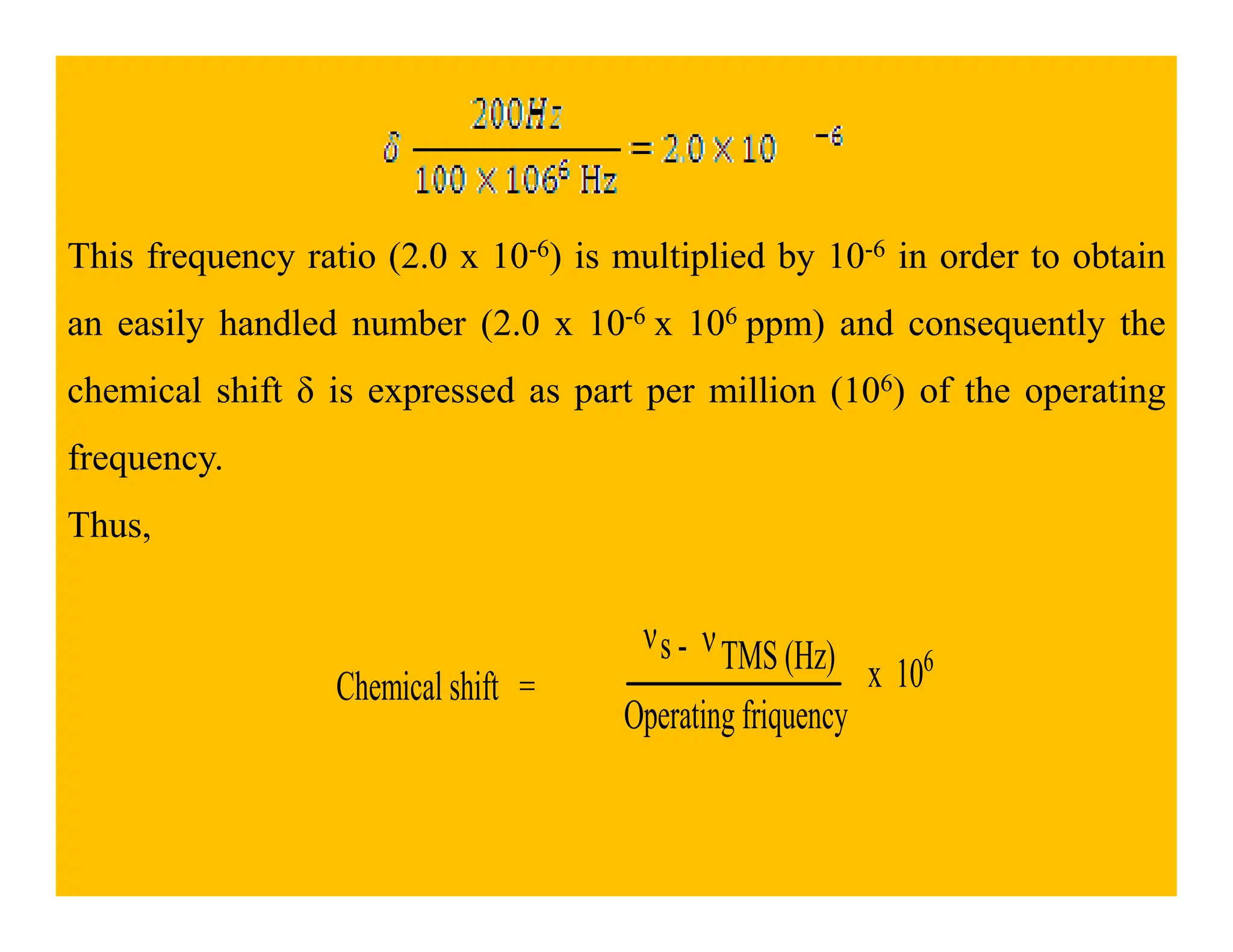
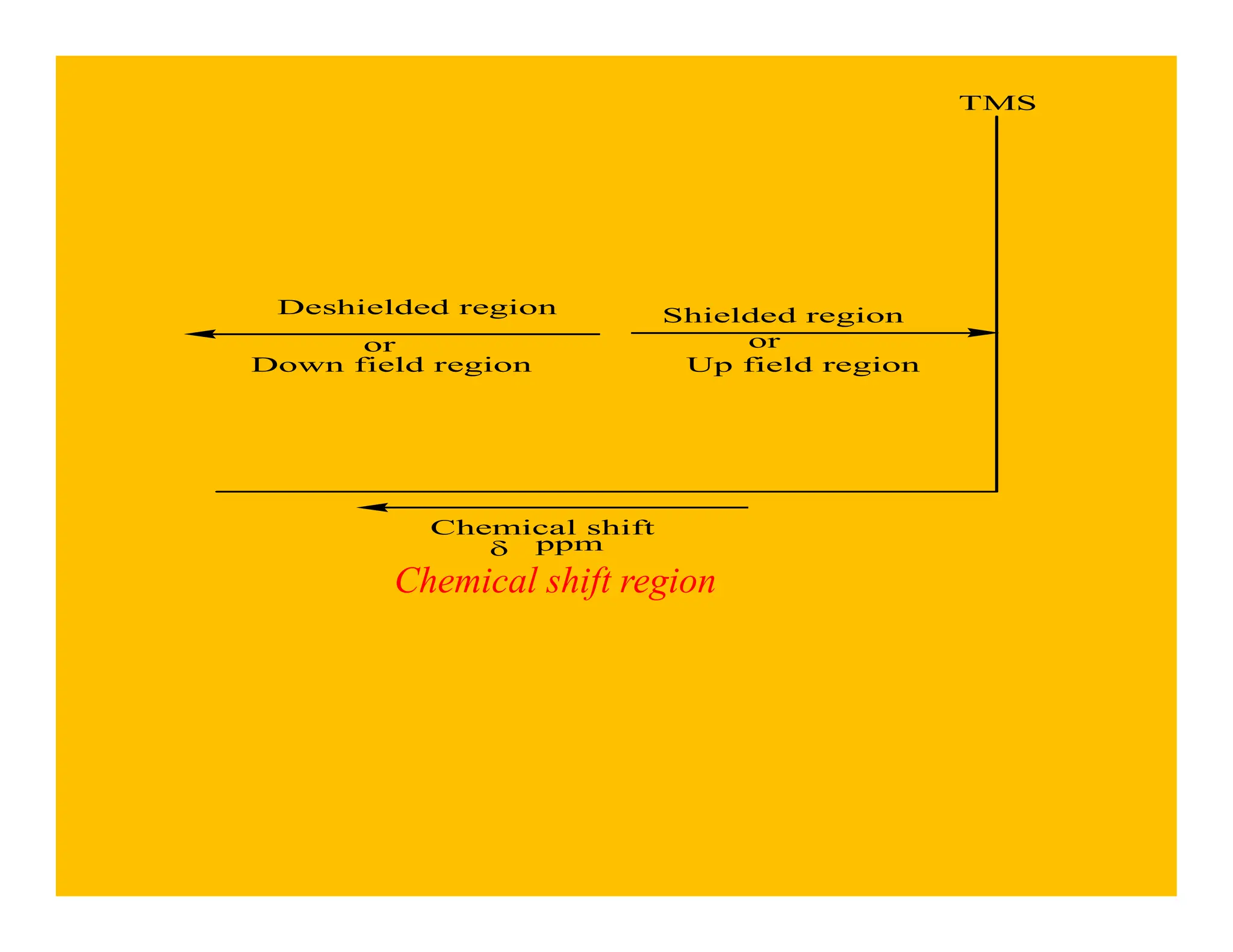
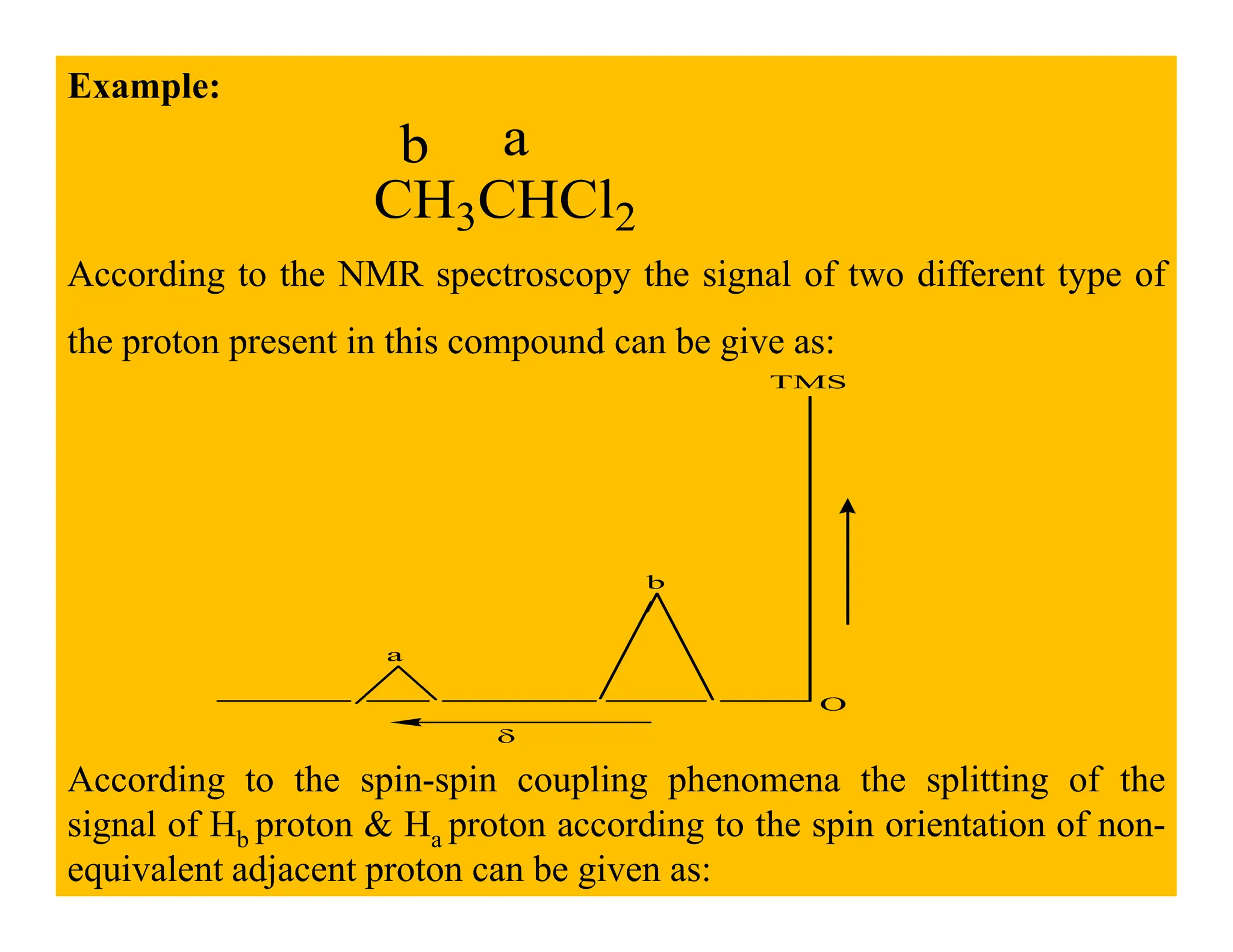
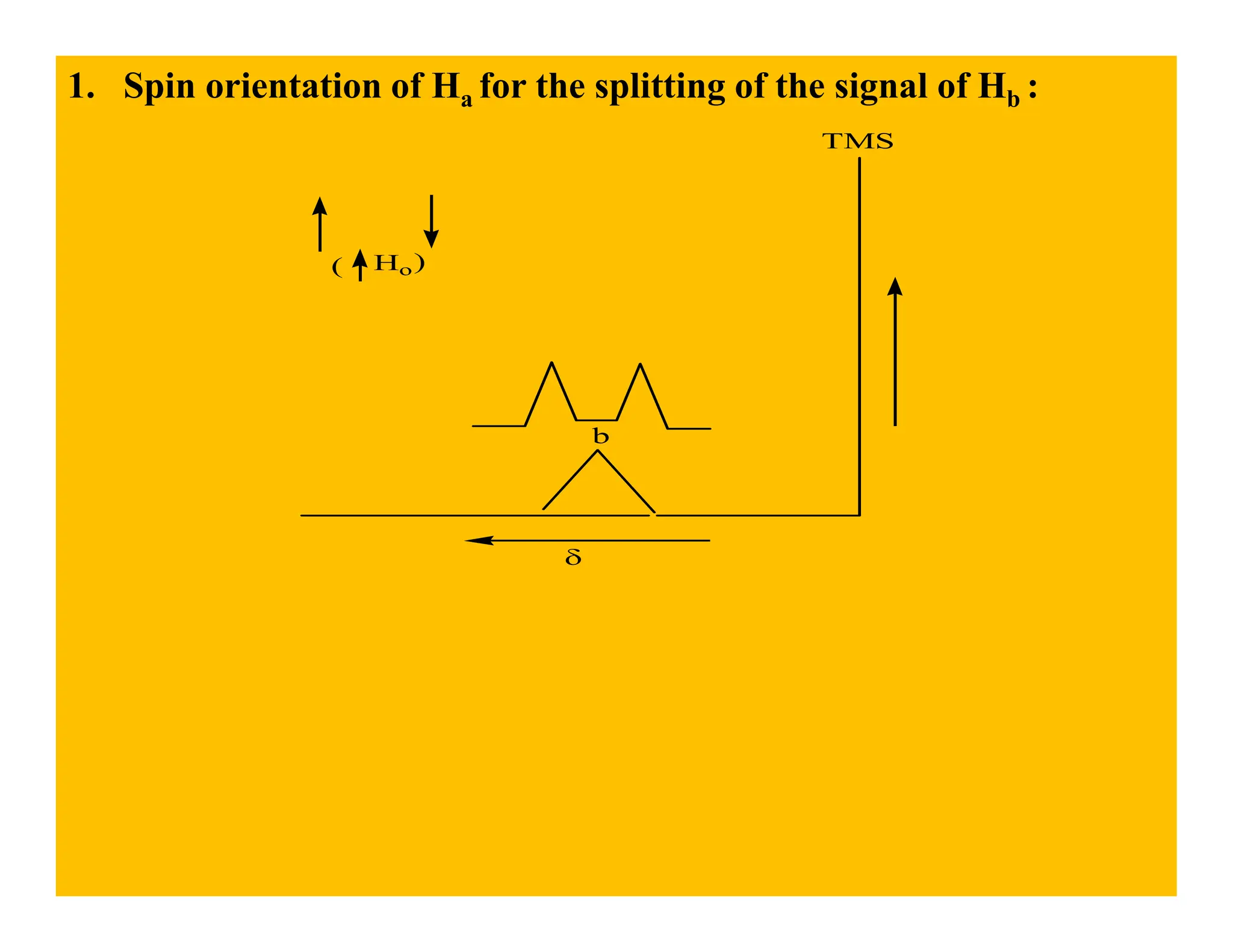
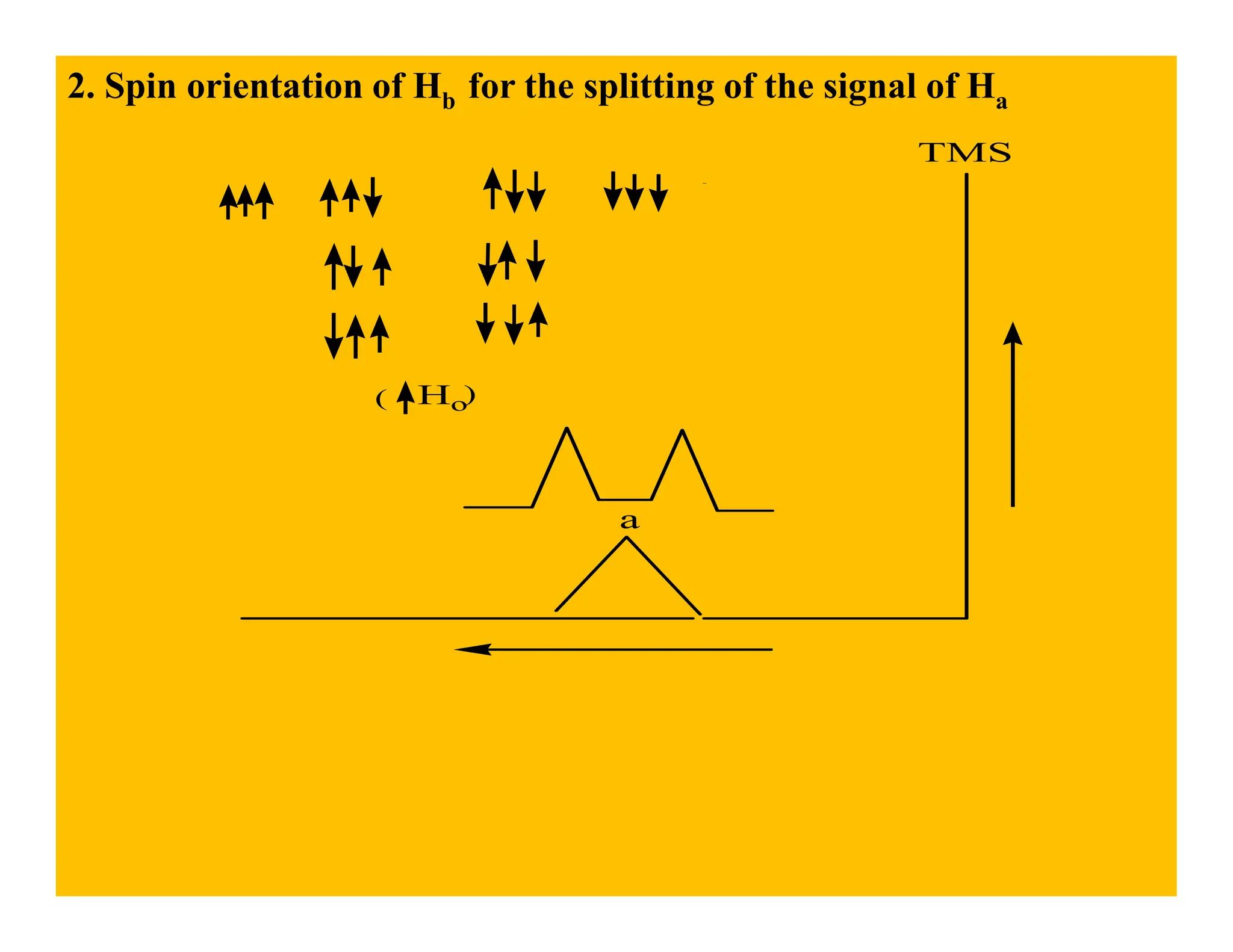
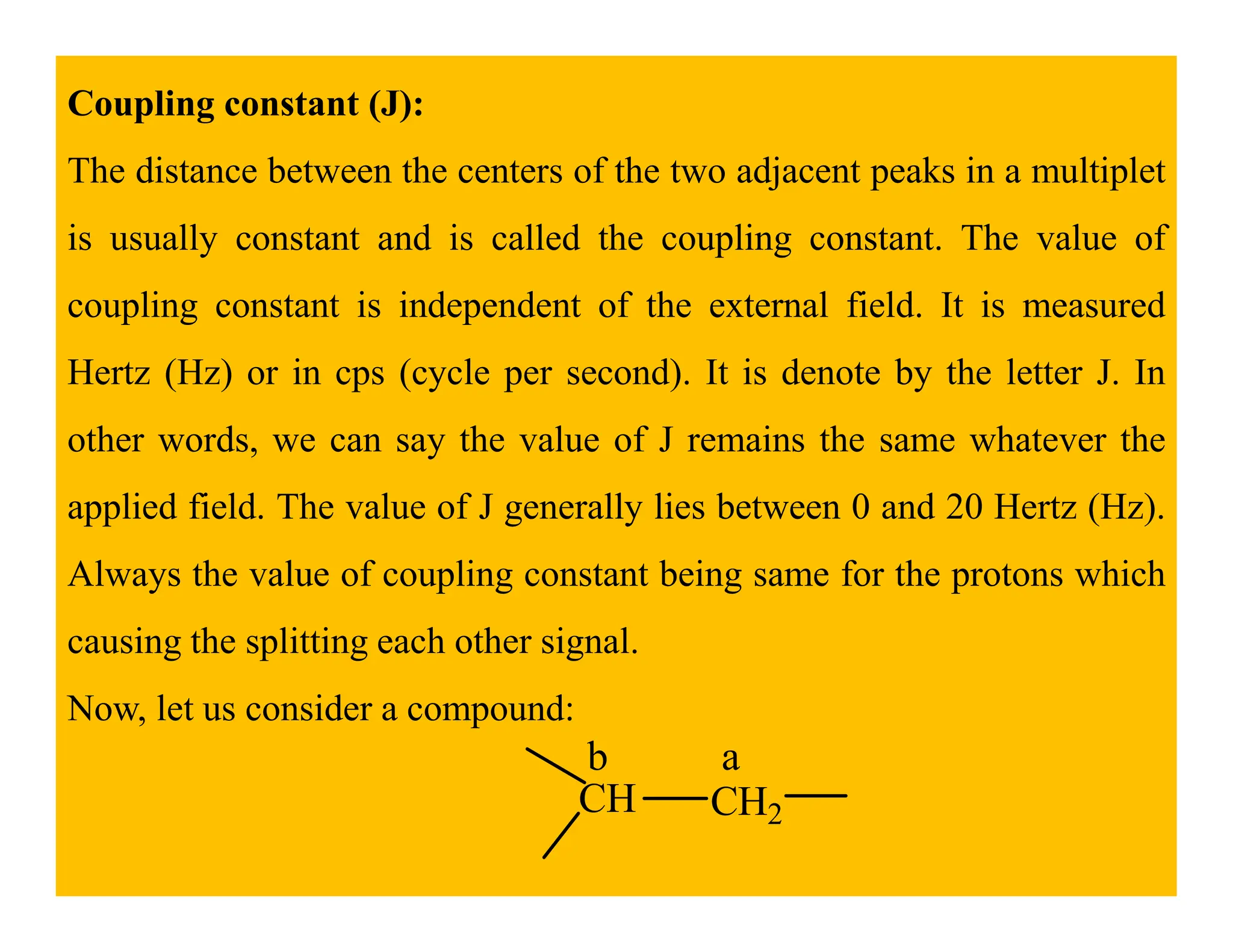
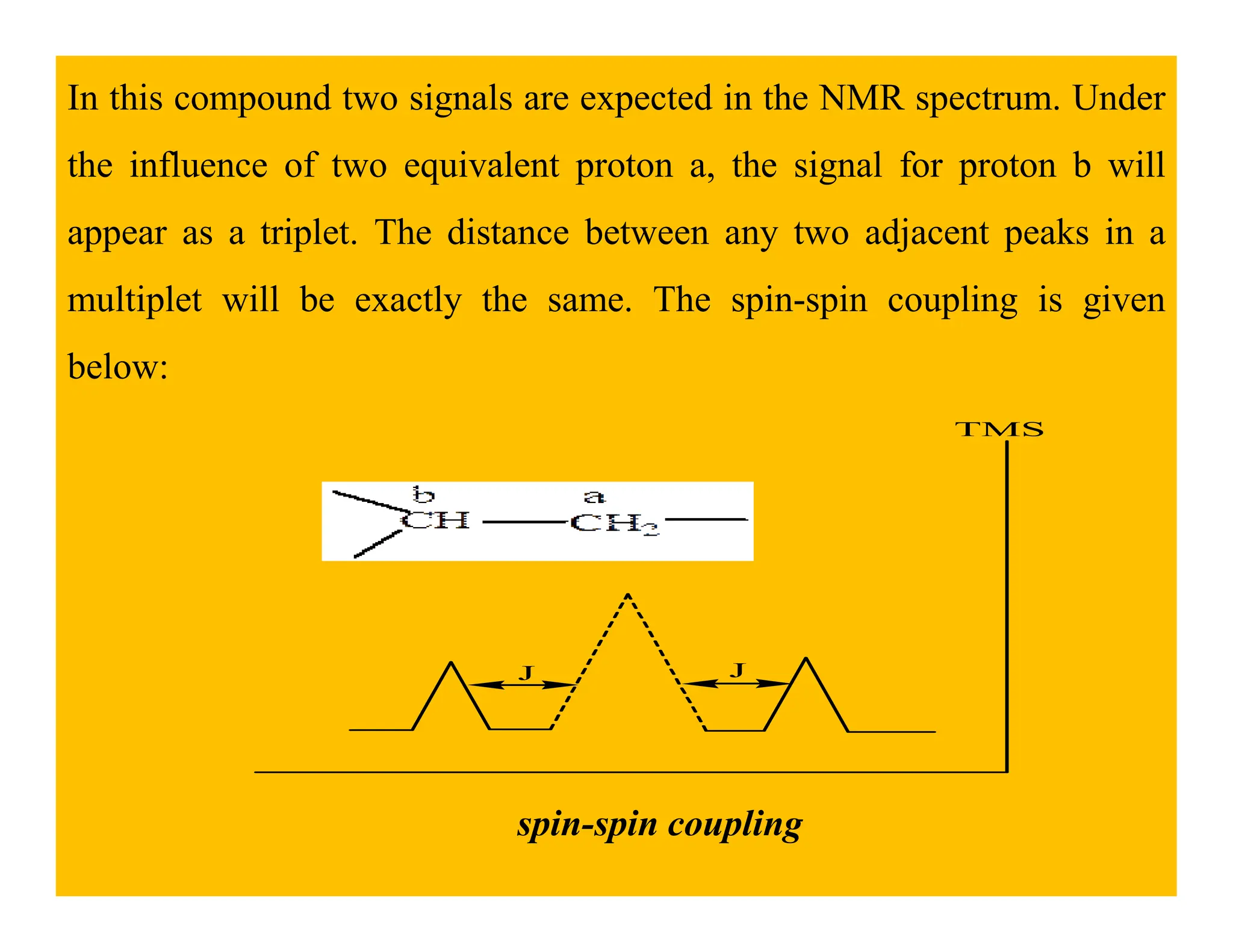
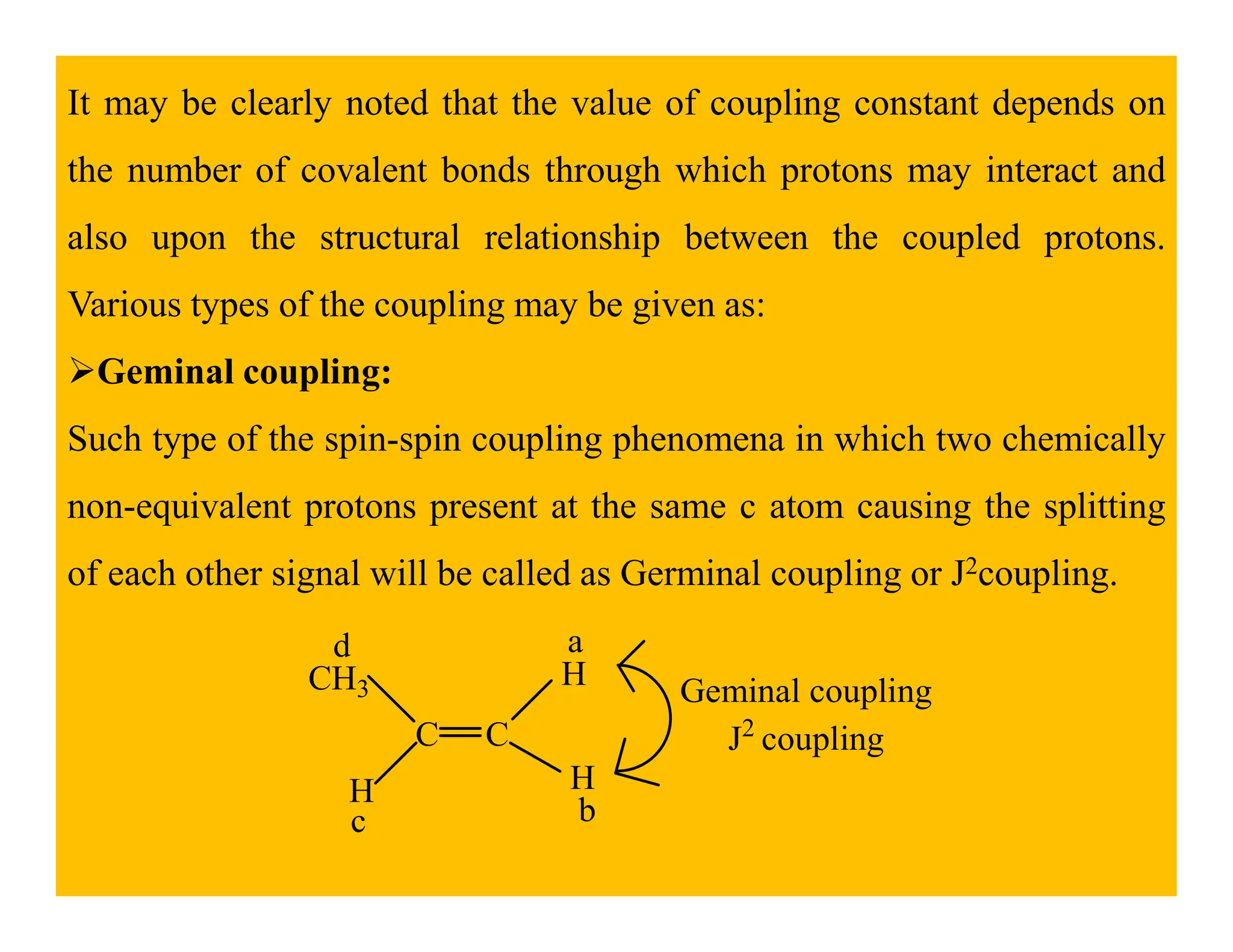
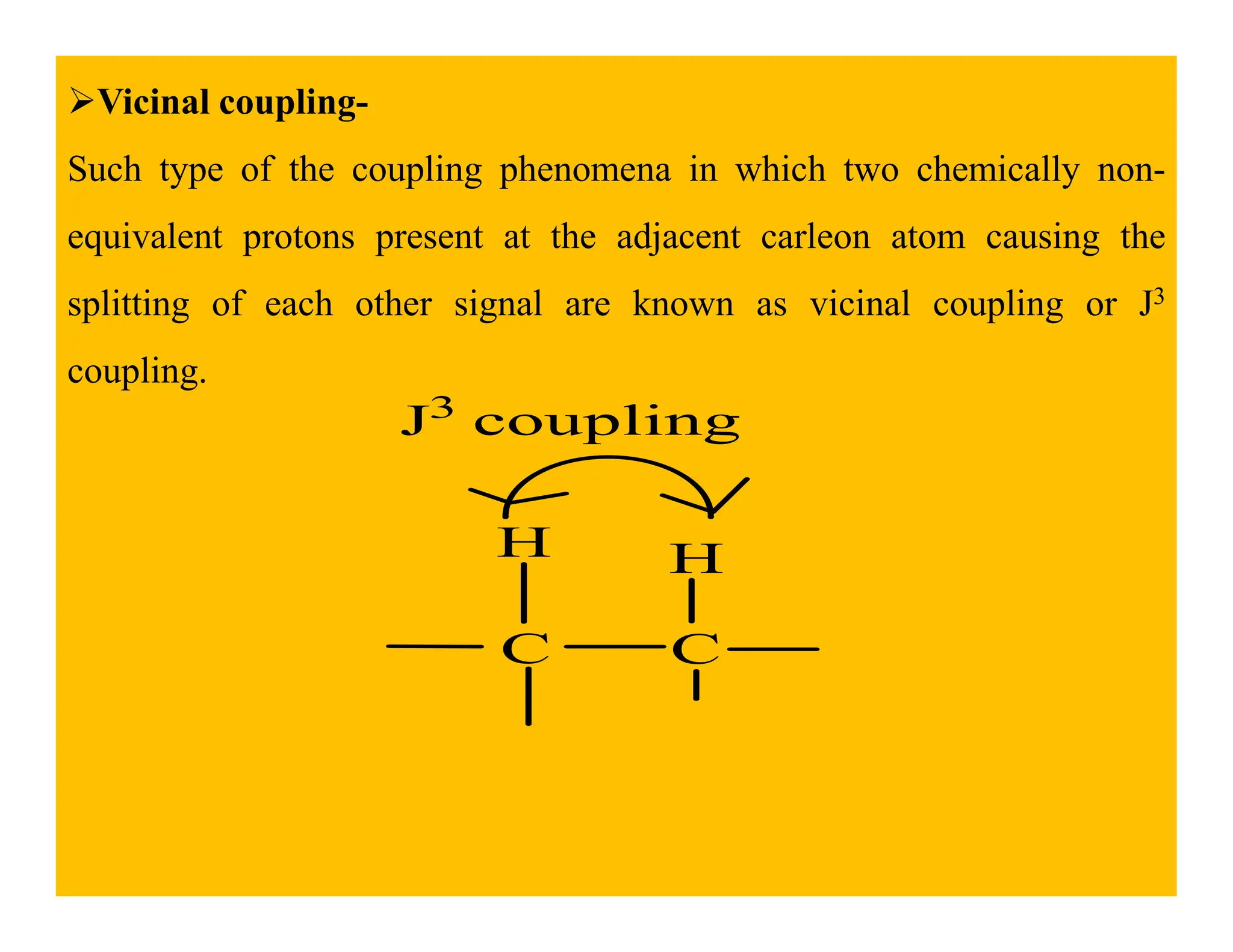
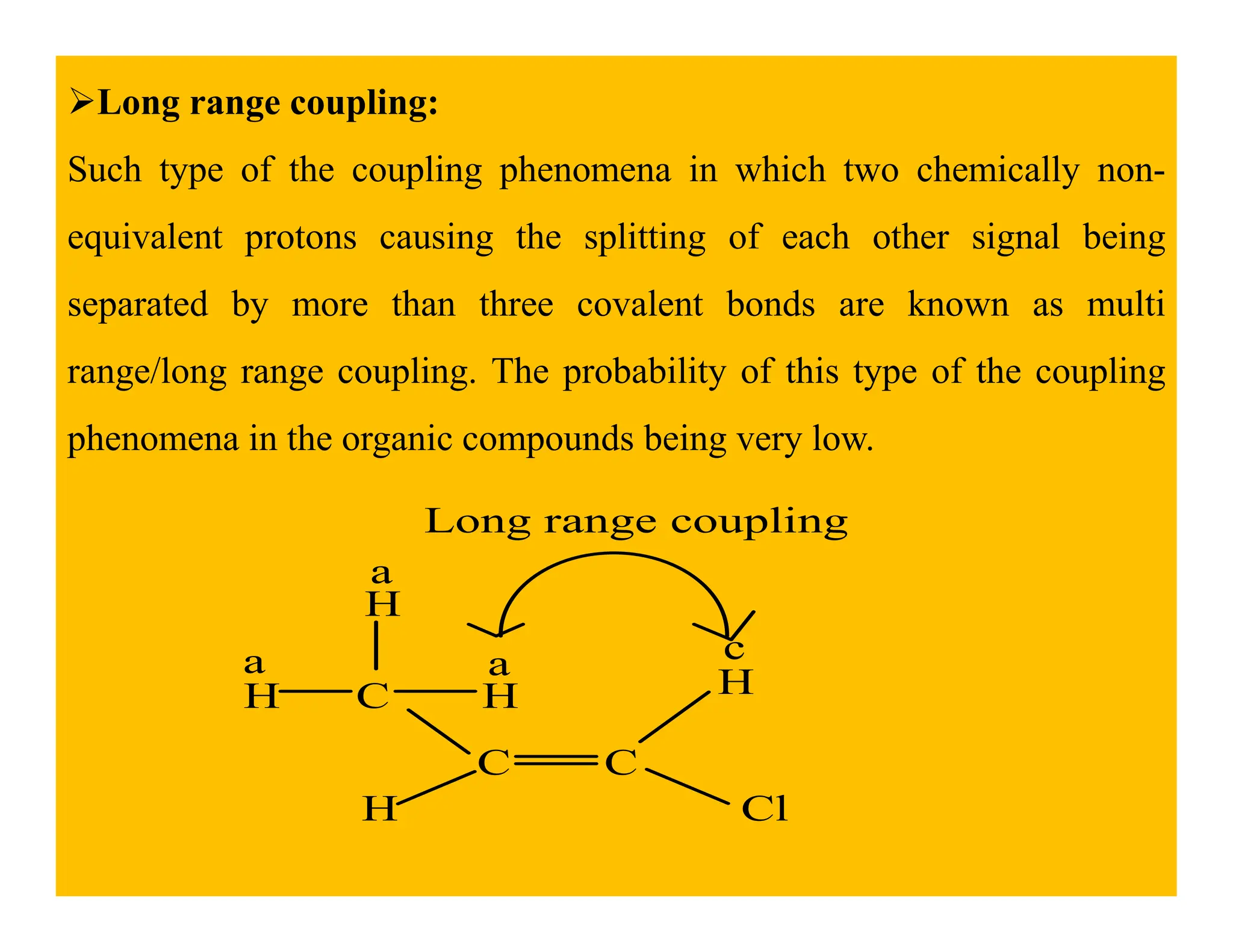
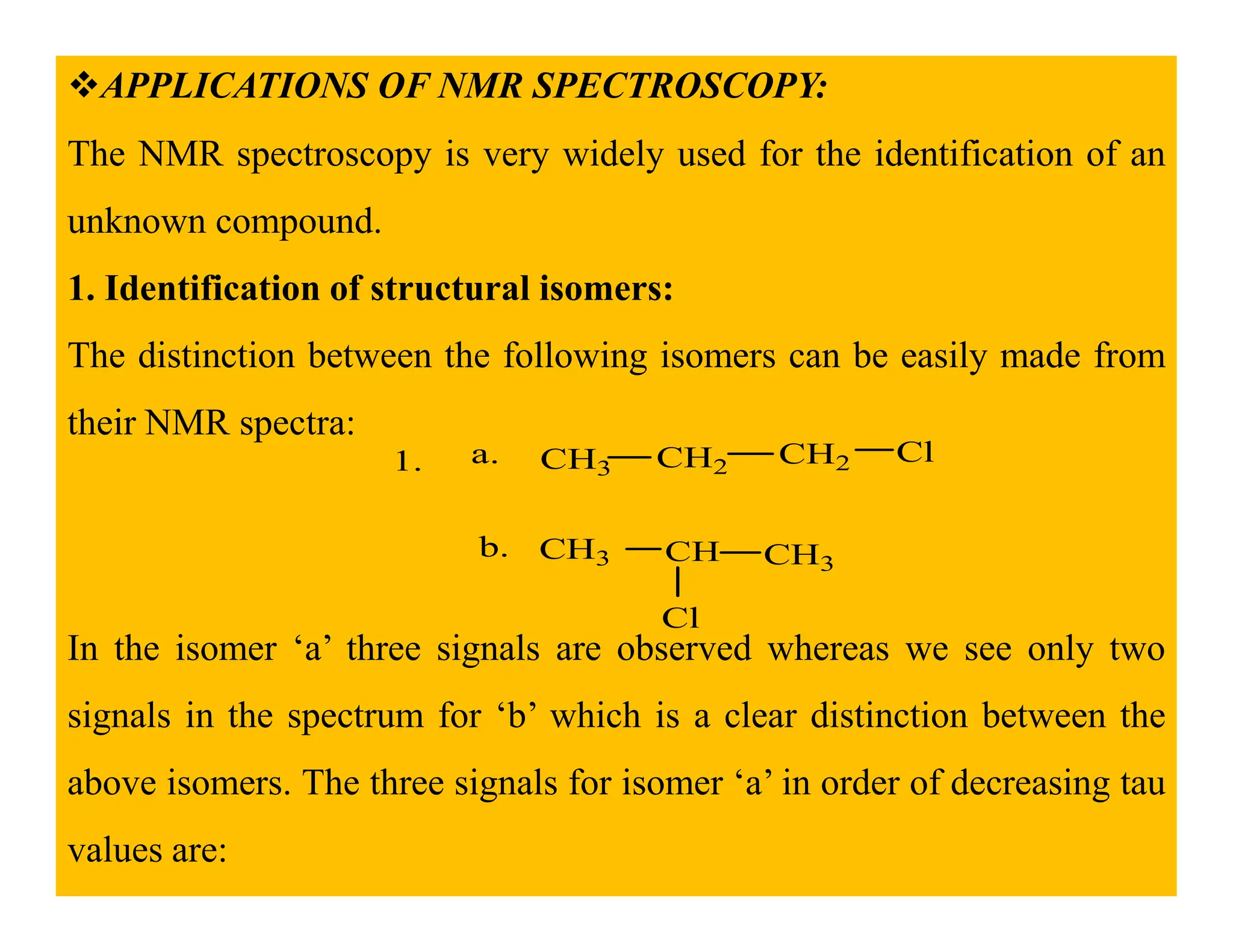
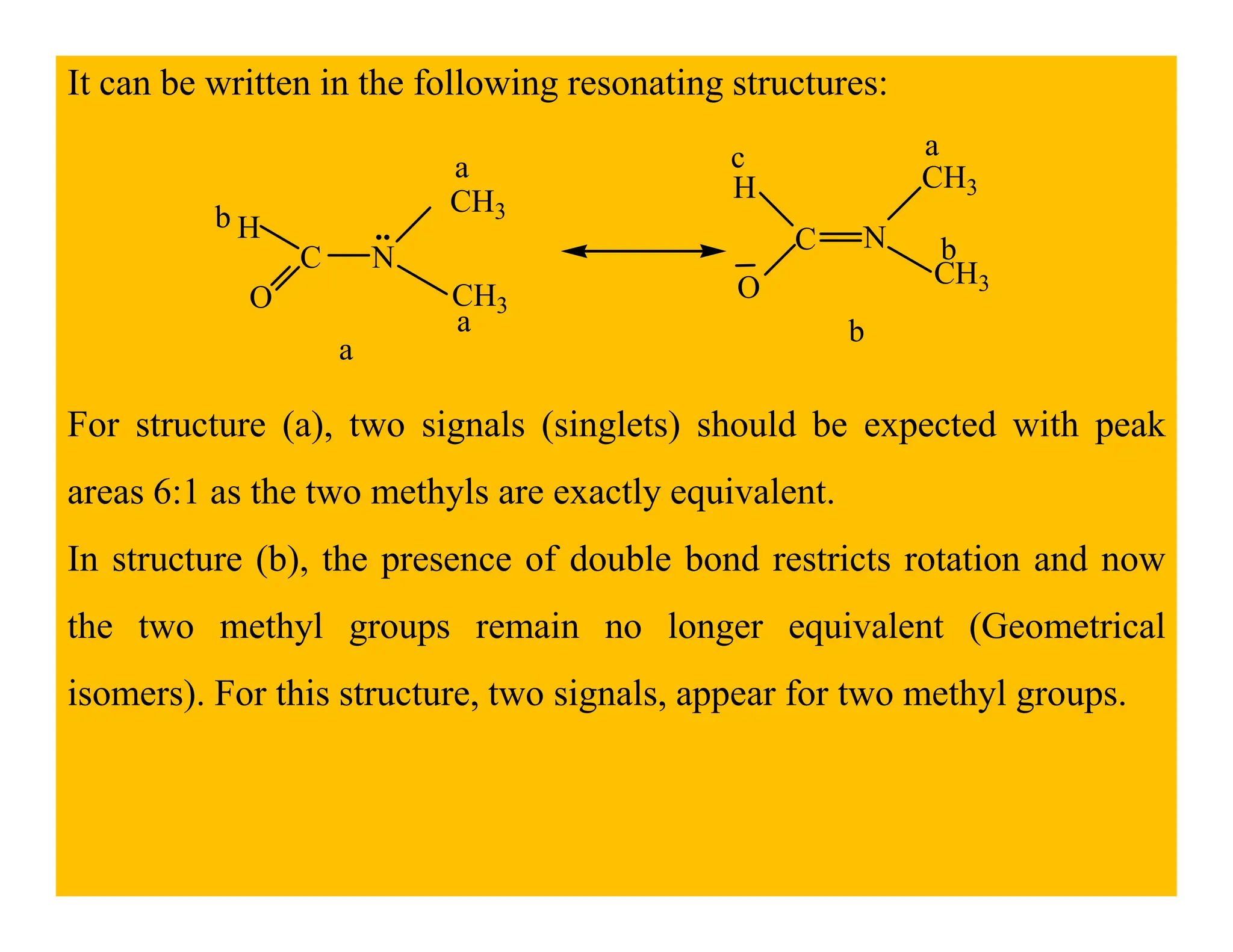
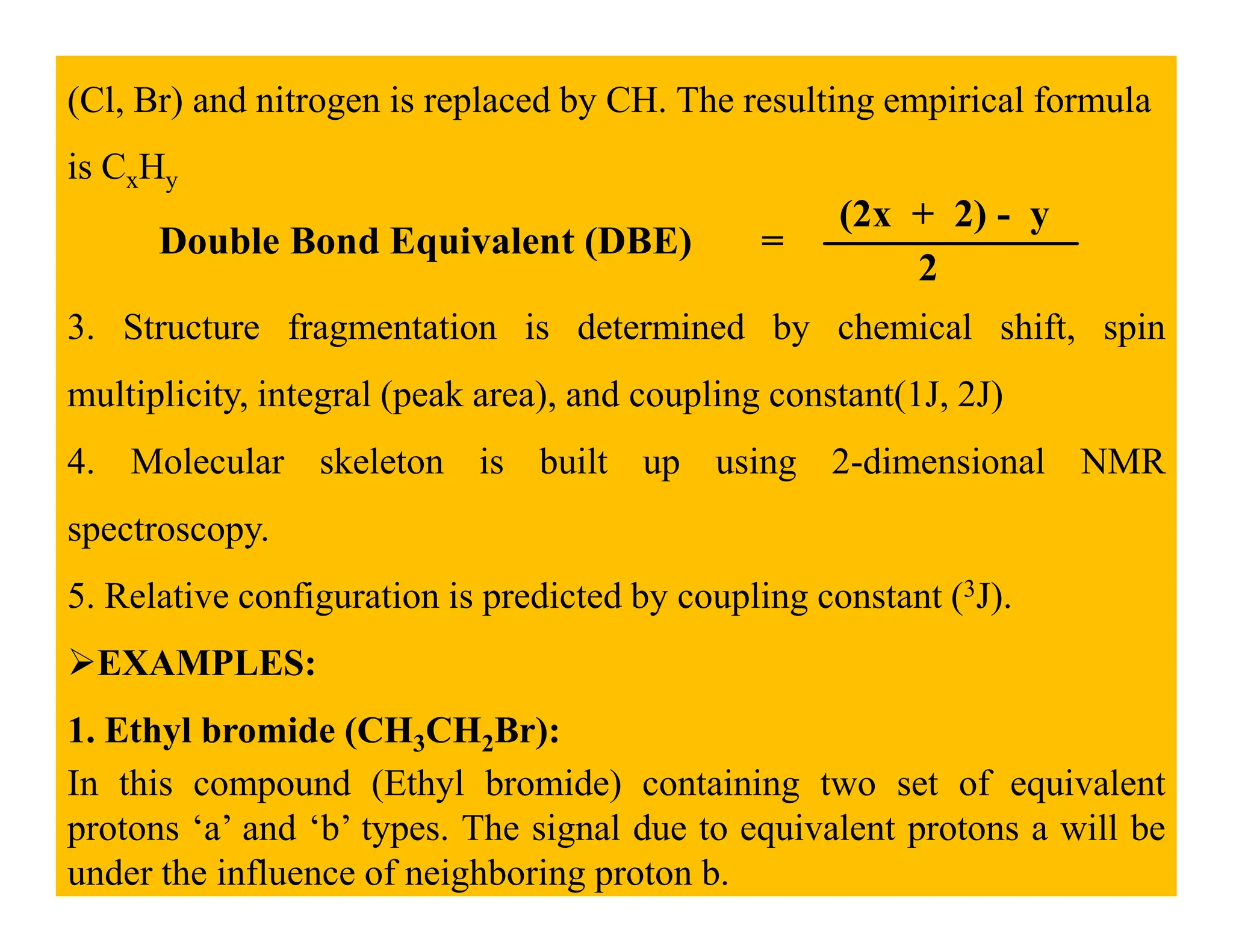
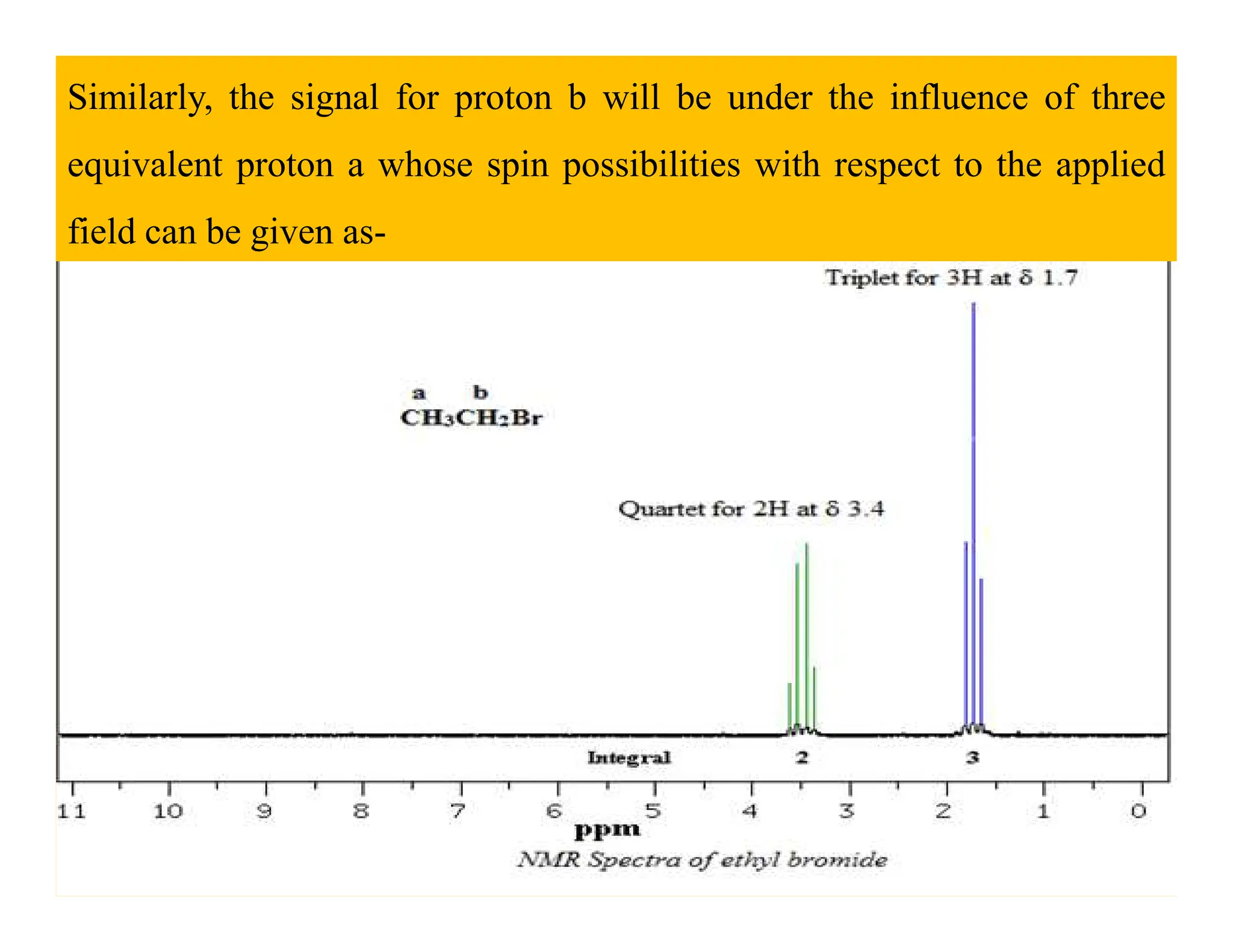
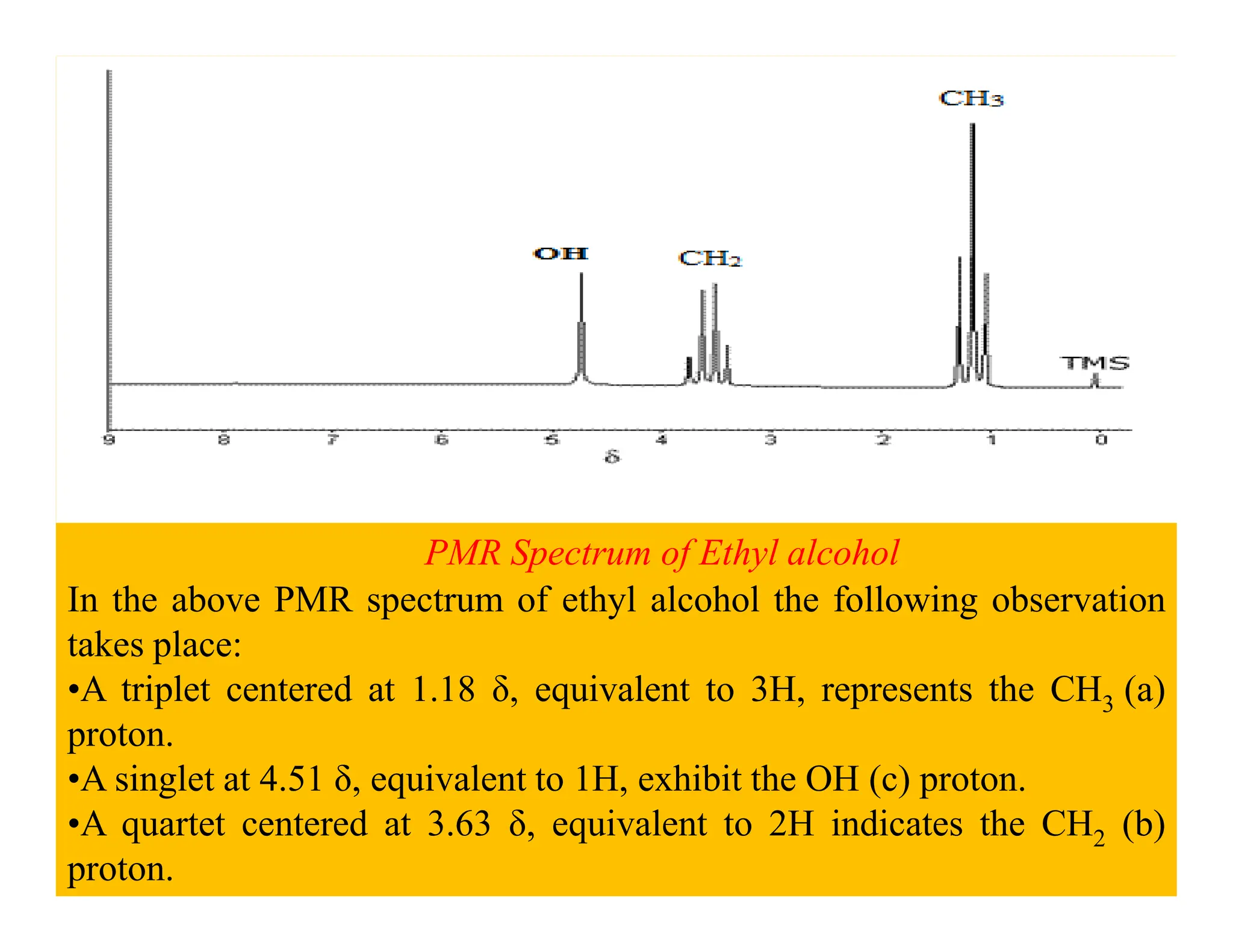
This document provides an introduction to NMR spectroscopy. It discusses key concepts such as NMR active nuclei, resonance and relaxation phenomena, nuclear shielding and deshielding, chemically equivalent and non-equivalent protons, chemical shift, spin-spin splitting and coupling constants. It also outlines some applications of NMR spectroscopy such as distinguishing structural isomers and detecting hydrogen bonding. The document concludes by discussing factors that are important for interpreting 1H NMR spectra such as chemical shift, spin multiplicity, coupling constants and integration.