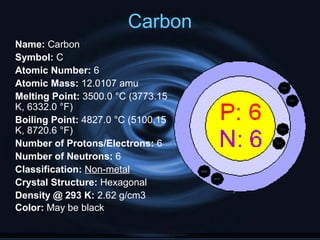
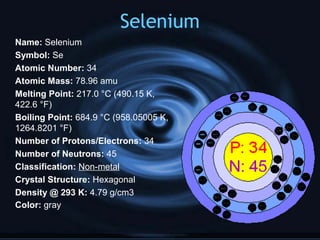
Non-metals include elements in groups 14-16 of the periodic table. They are not good conductors of electricity or heat, are brittle, and exist as gases or solids at room temperature. Non-metals have no metallic luster, oxidation numbers of ±4, -3, or -2, and do not reflect light well. Hydrogen, carbon, nitrogen, oxygen, phosphorus, sulfur, and selenium are examples of non-metals described in the document with their atomic properties.