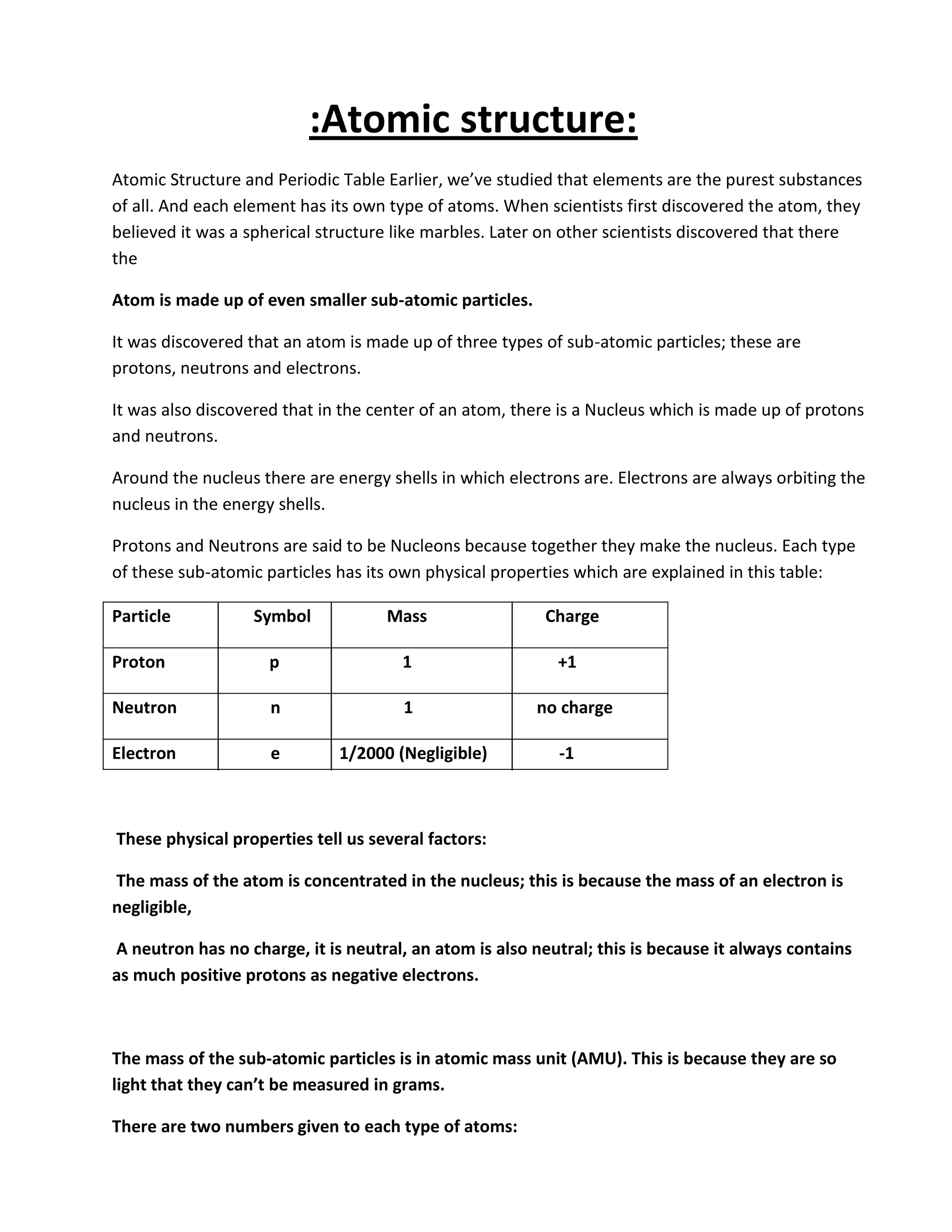
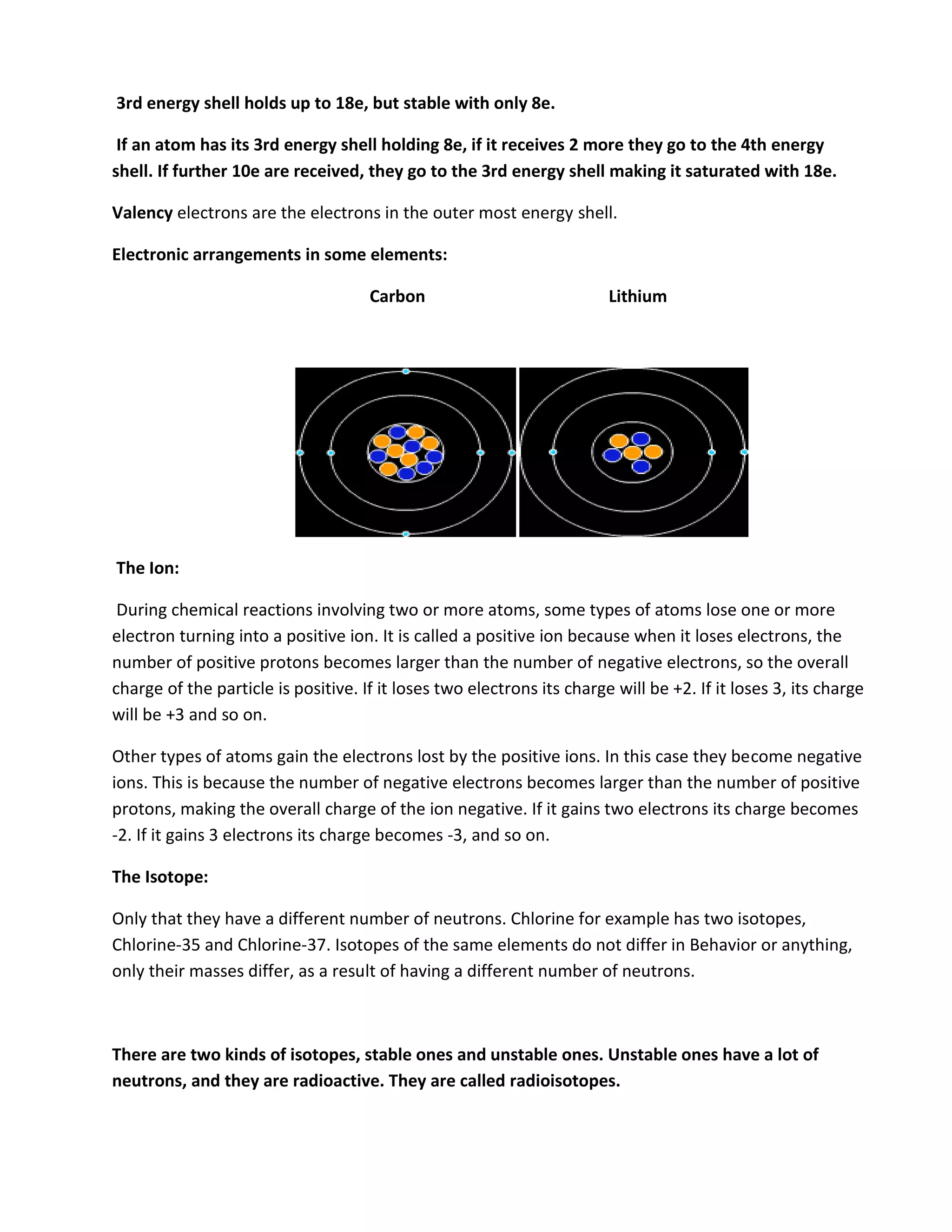
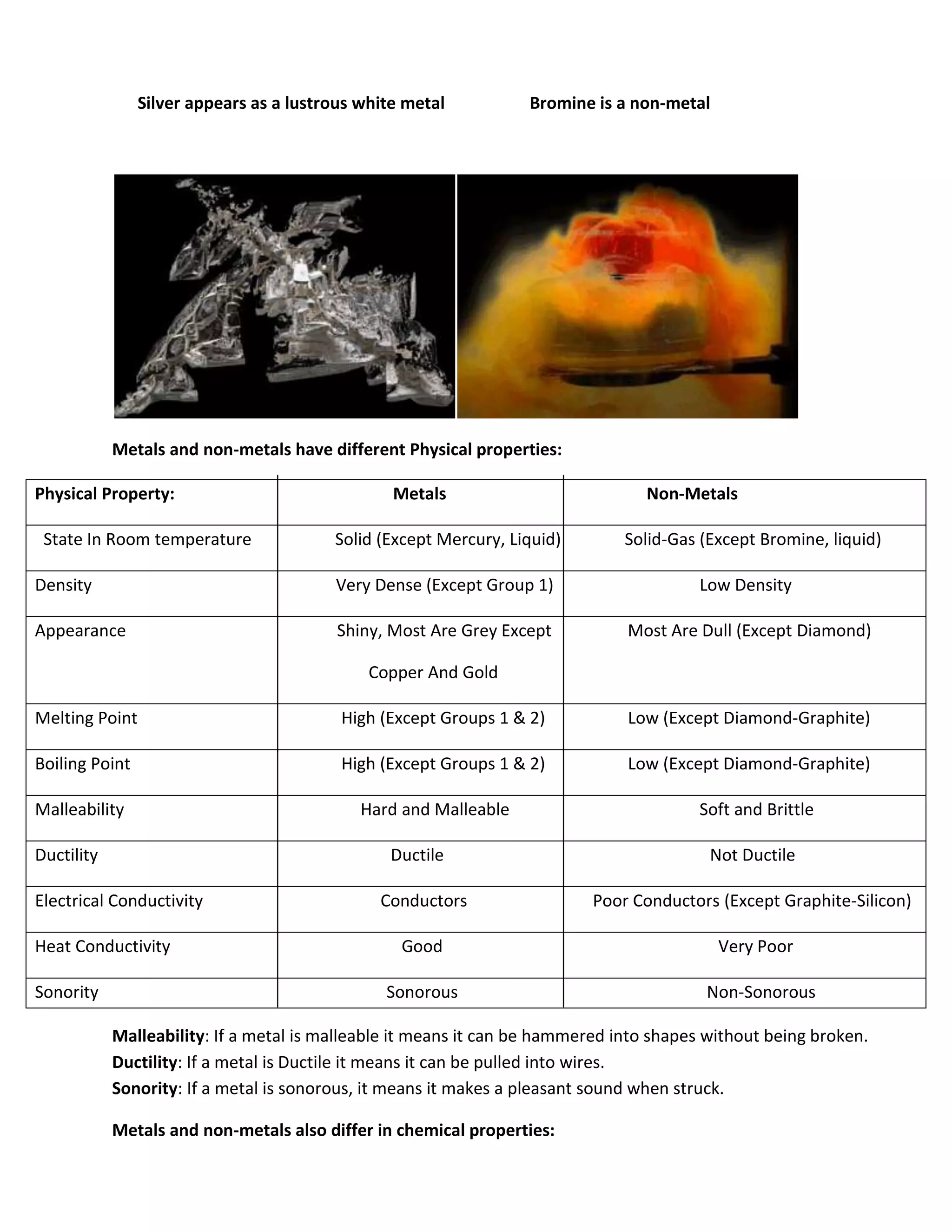
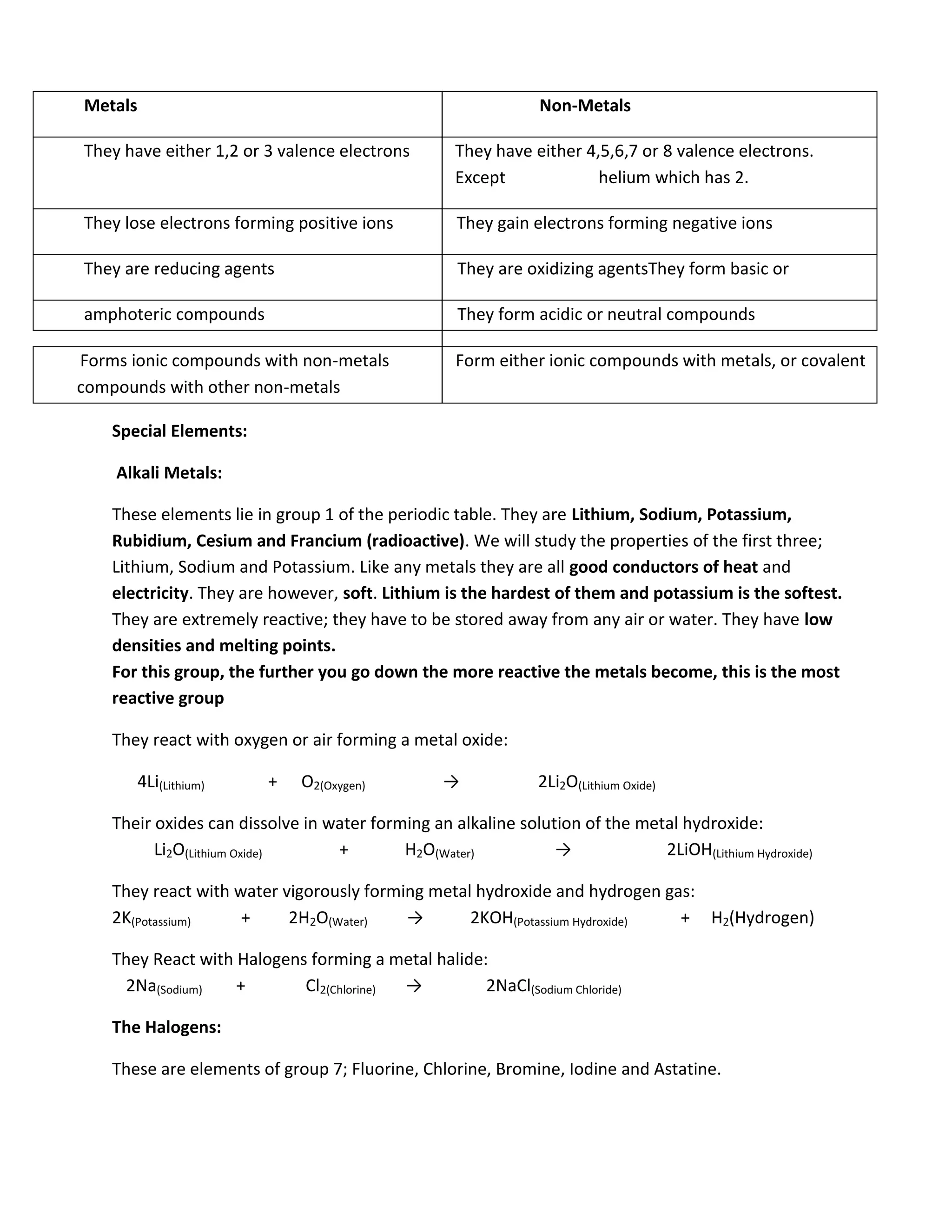
Atomic structure consists of subatomic particles including protons, neutrons, and electrons. Atoms have a nucleus composed of protons and neutrons surrounded by electrons in energy shells. The number of protons determines the element, while the number of neutrons distinguishes isotopes of that element. Elements exhibit trends in properties across periods and down groups in the periodic table due to their atomic structure and configuration of electrons.