Embed presentation
Downloaded 153 times

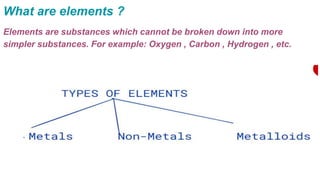
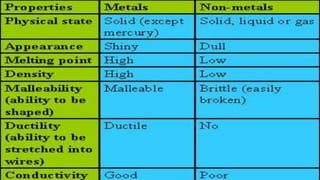










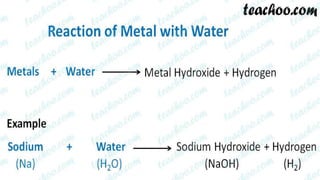



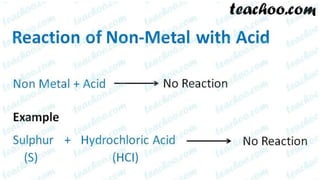
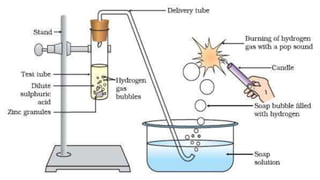
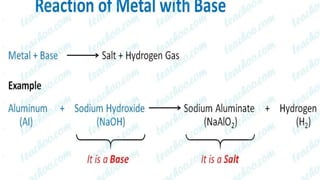

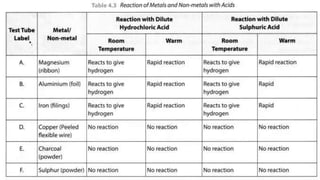


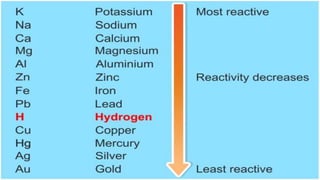




Metals and non-metals are two types of elements. Metals are shiny, sonorous, ductile, malleable, and good conductors of heat and electricity, while non-metals are dull, non-sonorous, non-ductile, non-malleable, and poor conductors. Some exceptions are that mercury is the only metal in liquid form, sodium and potassium are soft metals, iodine is the only lustrous non-metal, and graphite conducts electricity unlike most non-metals. Metals react with oxygen to form metal oxides, hydroxides, and carbonates, while non-metals react with bases in a more complex process without hydrogen gas




























