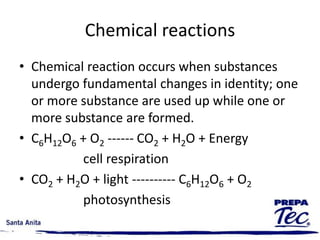
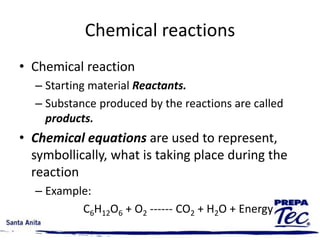
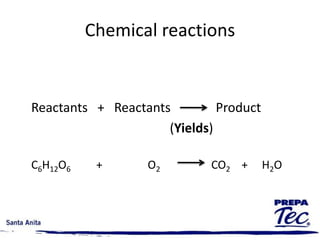
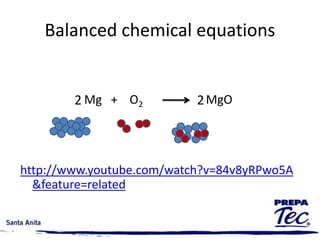
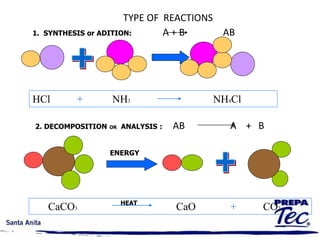
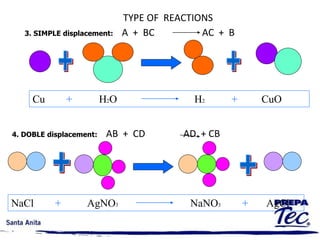
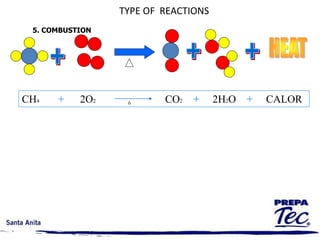
Chemical reactions involve substances undergoing fundamental changes in identity, with reactants being used up and products being formed. Two key examples are cell respiration, where glucose and oxygen react to produce carbon dioxide, water, and energy, and photosynthesis, where carbon dioxide, water, and light energy are used to produce glucose and oxygen. Chemical reactions are represented by balanced chemical equations that show the reactants and products. There are several types of chemical reactions including synthesis, decomposition, single displacement, double displacement, and combustion.