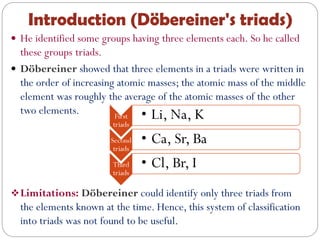
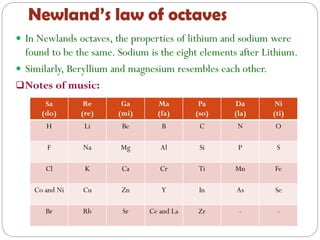
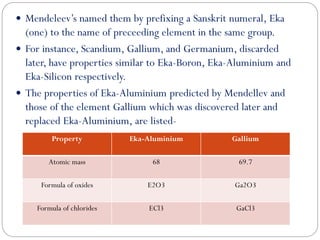
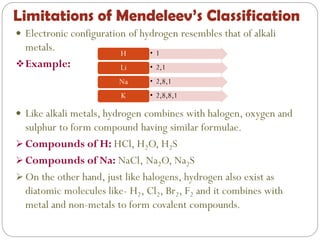
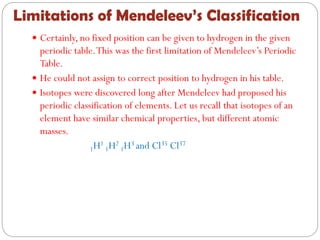
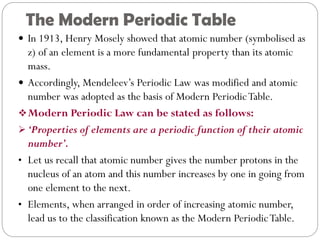
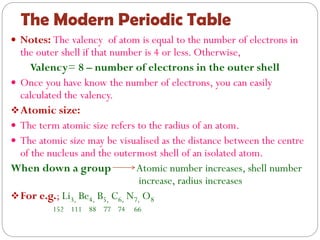
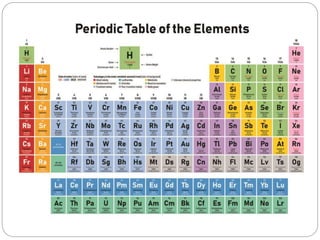
The document provides a comprehensive overview of the periodic classification of elements, highlighting key attempts such as Döbereiner's triads, Newland's law of octaves, and Mendeleev's periodic table, culminating in the adoption of atomic number as the basis for the modern periodic table. It details the historical context, significant contributions, and limitations of each classification system. Additionally, it discusses trends such as atomic size and metallic/non-metallic properties within the modern periodic framework.