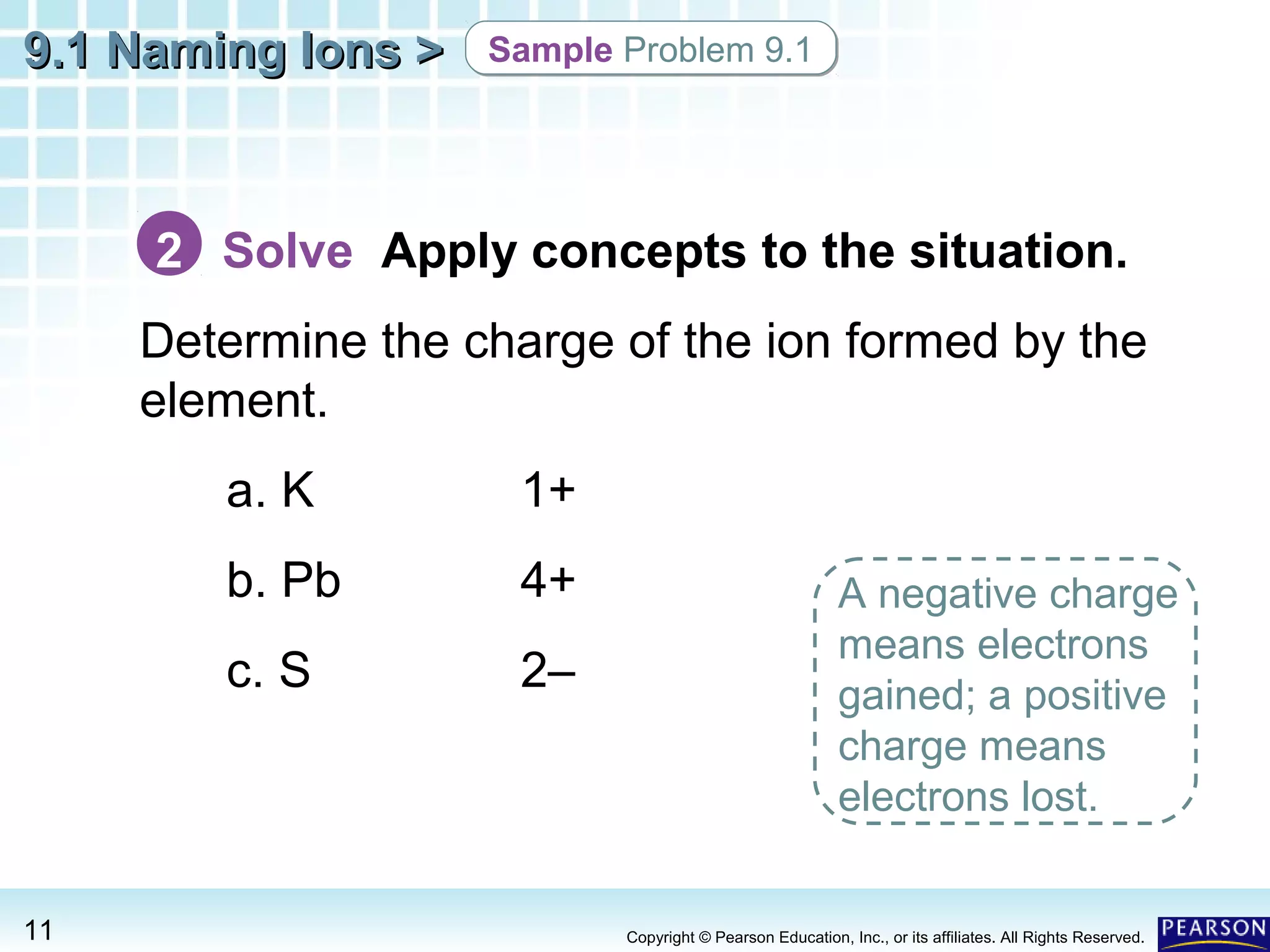
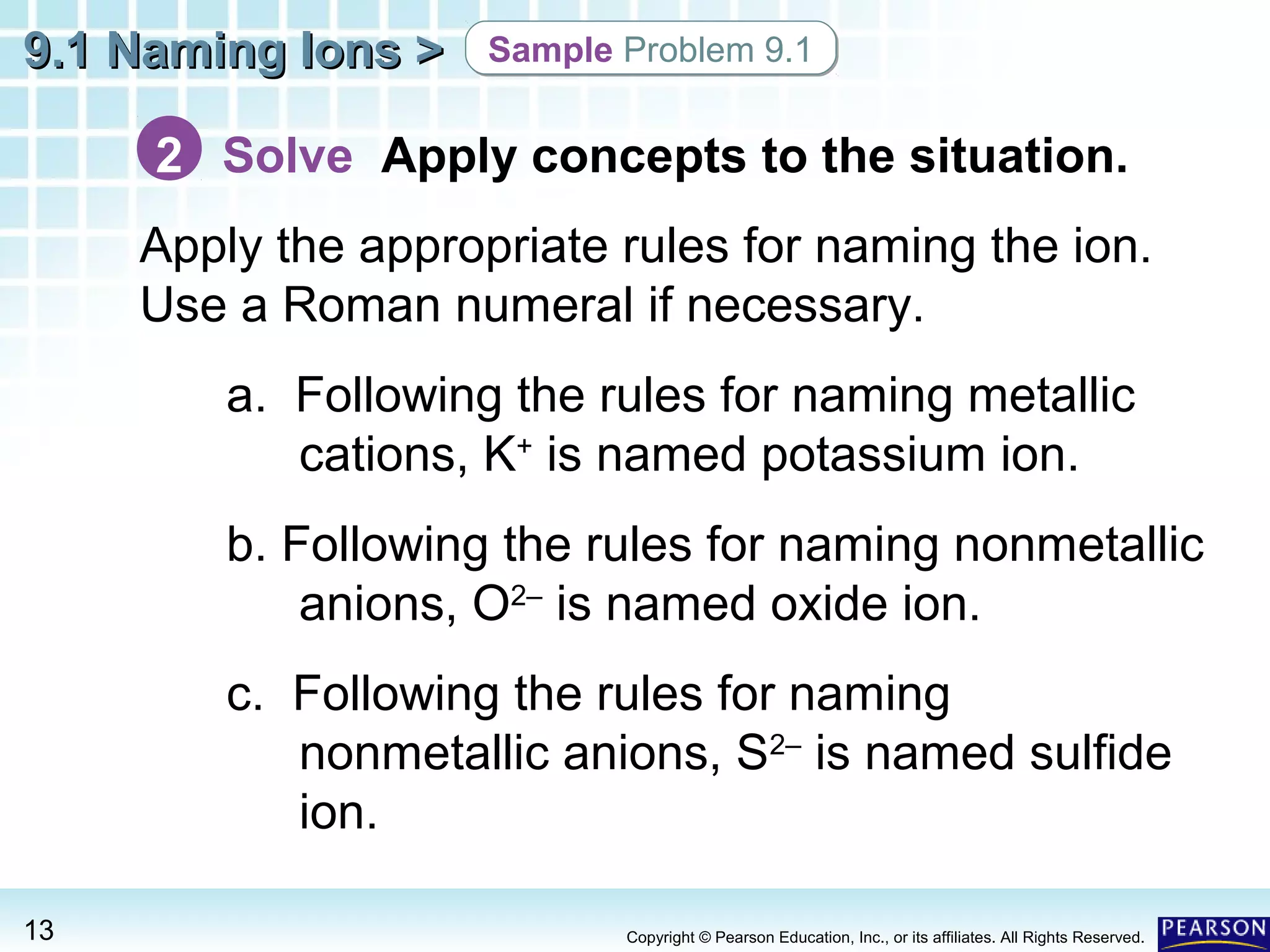
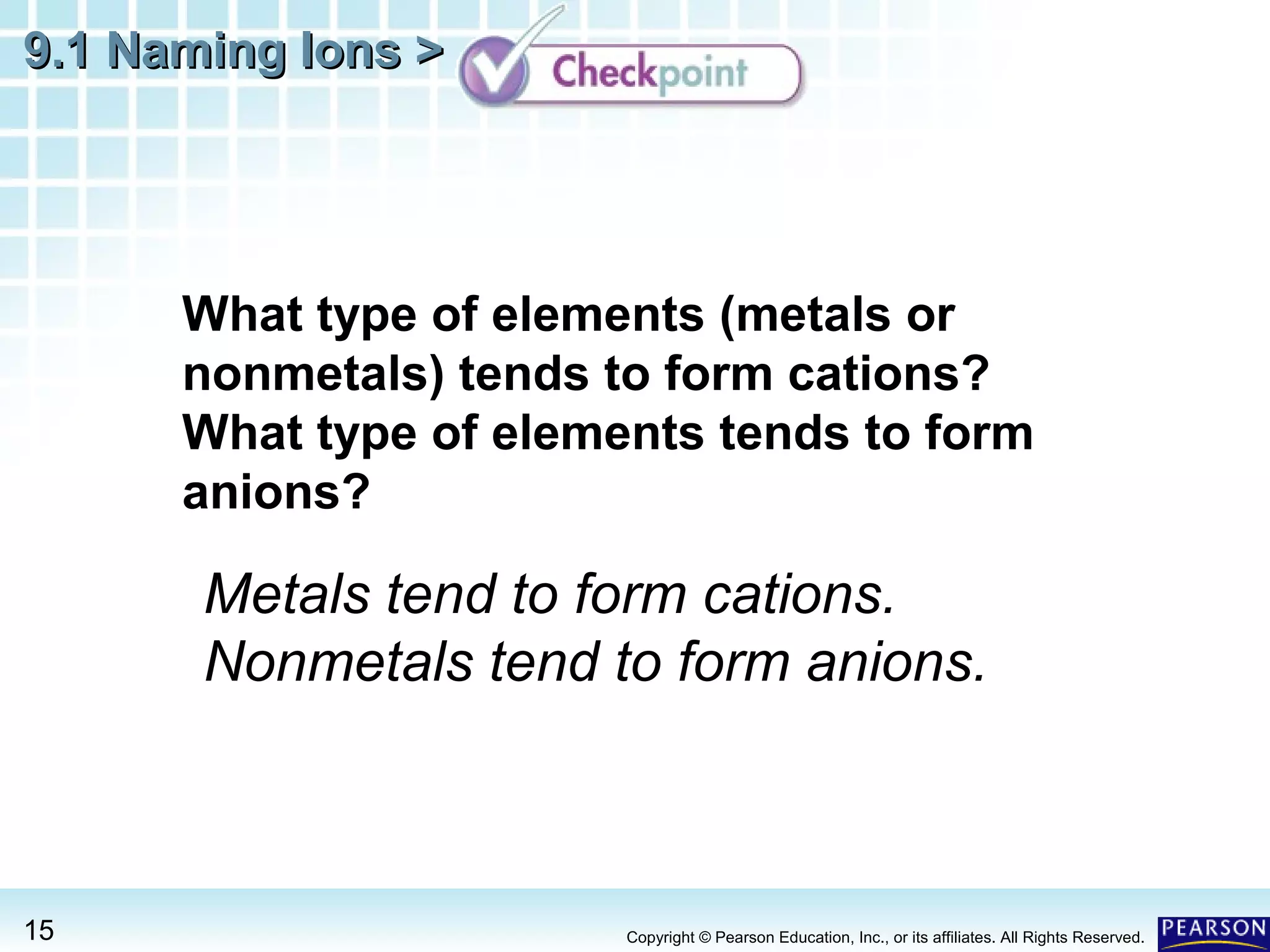
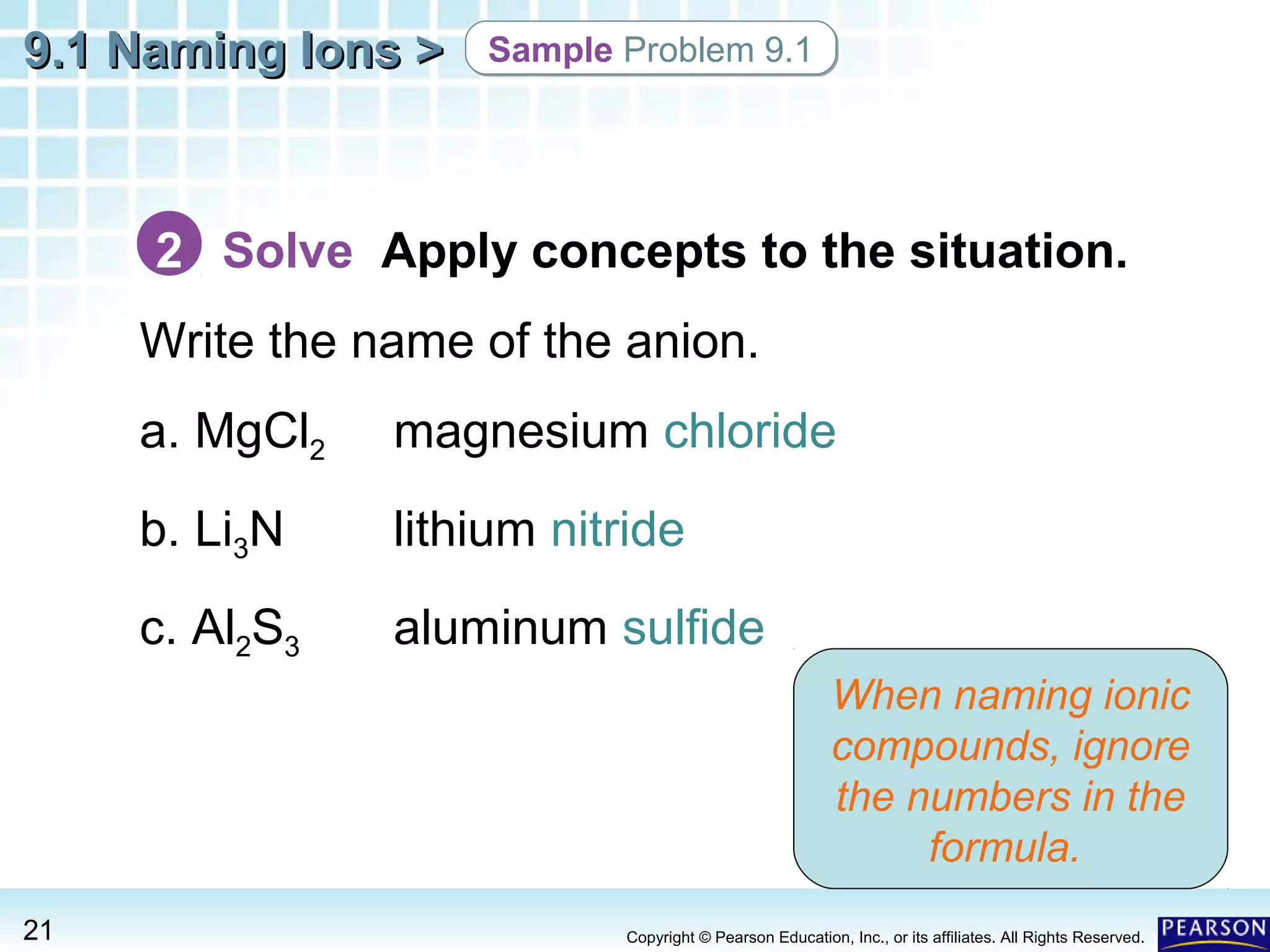
The document discusses naming ions and ionic compounds. It explains that ions are formed when atoms gain or lose electrons to obtain full outer shells, becoming negatively or positively charged anions and cations. Cations are named after the metal element, while anions are named by adding -ide to the nonmetal element name. Examples are provided of naming common monatomic cations such as sodium (Na+) and calcium (Ca2+), and anions such as chloride (Cl-) and sulfate (SO42-). The document concludes by demonstrating how to name ionic compounds by combining the names of the respective ions.