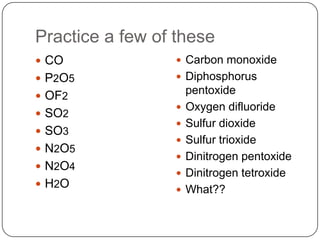
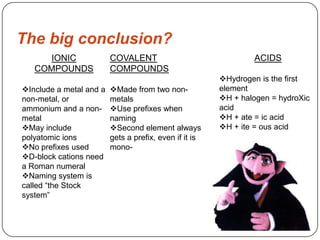
This document provides information on naming covalent compounds and acids. It discusses how to identify covalent compounds based on their composition and discusses prefixes used in naming them such as mono, di, and tri. Rules are provided for determining the order and modified ending of elements in covalent compound names. Examples of naming covalent compounds like CO, P2O5, and H2O are also provided. The document also discusses naming acids based on whether they contain halides, -ates, or -ites and the number of hydrogens added to match charge. It concludes with a comparison of naming conventions for ionic compounds, covalent compounds, and acids.