Embed presentation
Downloaded 39 times


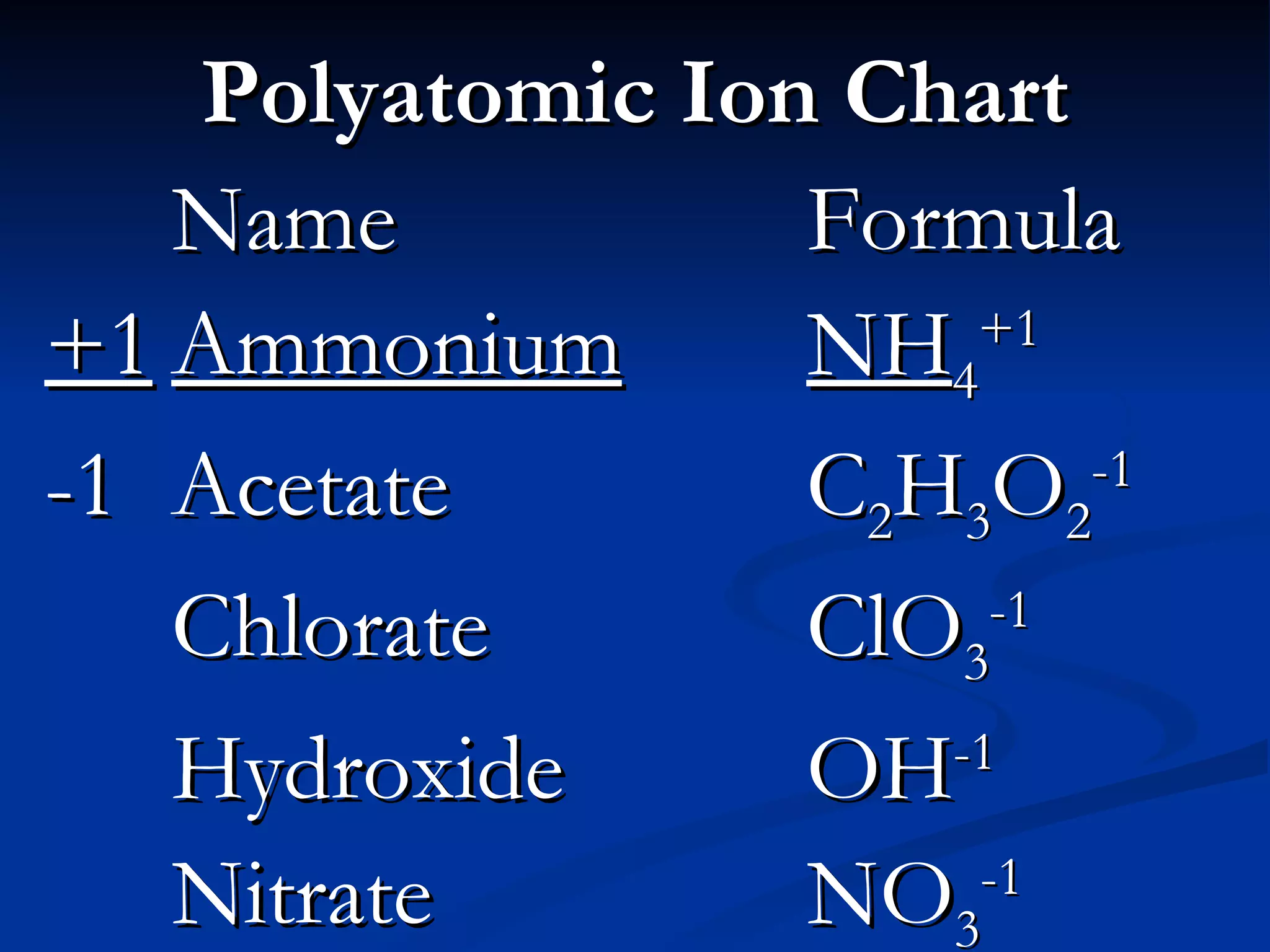
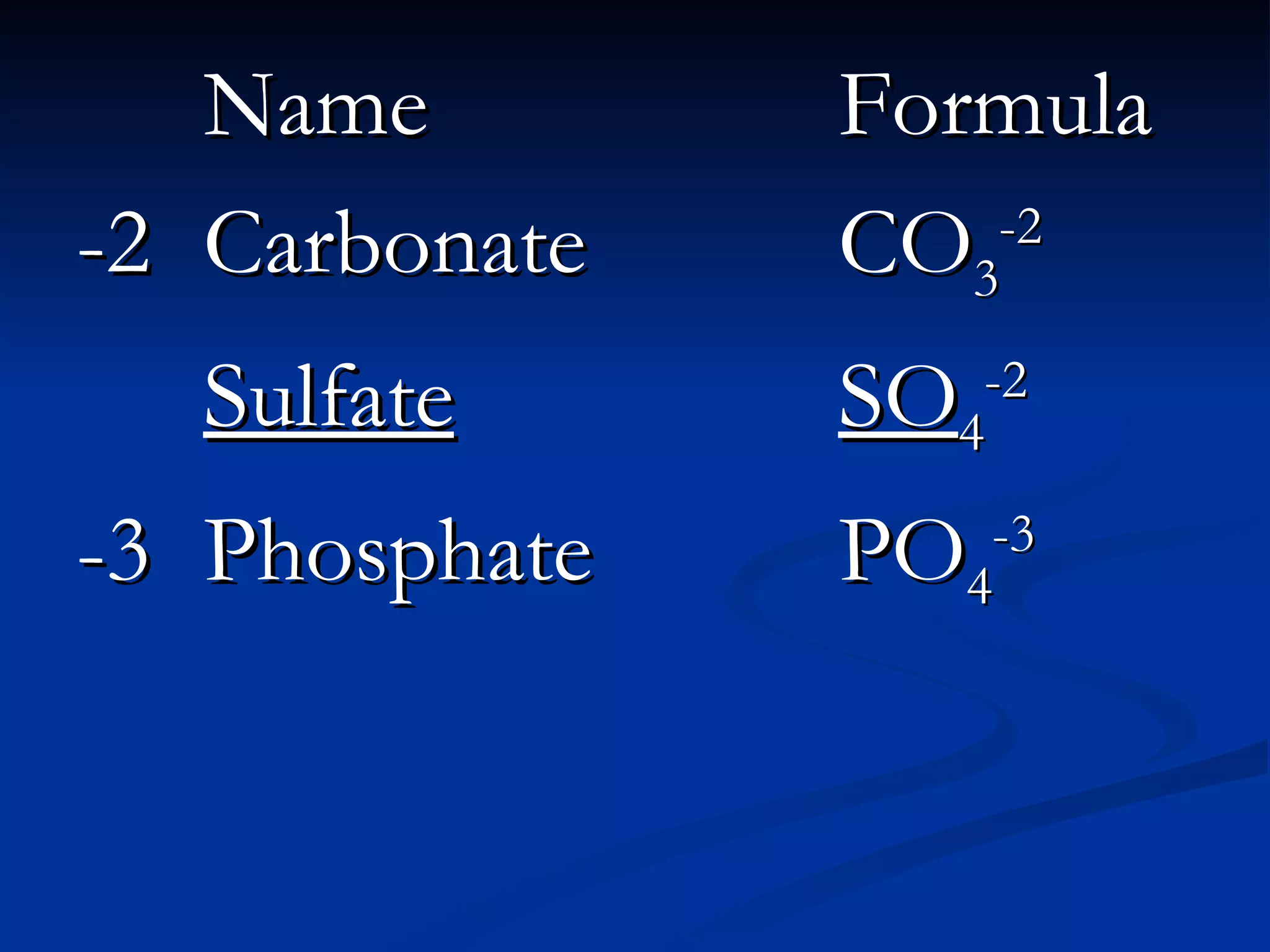






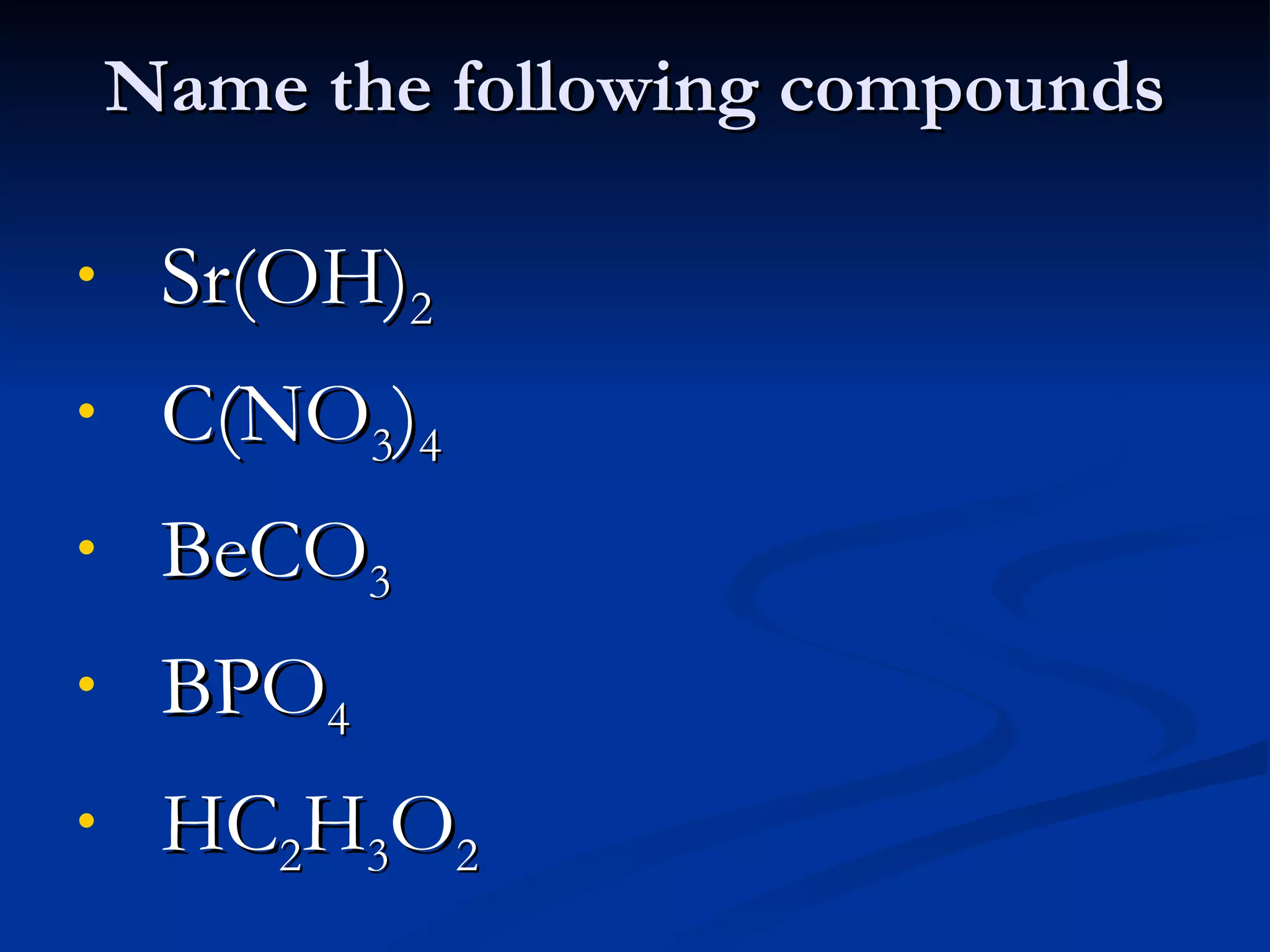
This document discusses polyatomic ions, which are positively or negatively charged groups of covalently bonded atoms. It provides examples of common polyatomic ions like ammonium, acetate, chlorate, and lists their formulas. It then outlines the steps to write formulas and name compounds that contain polyatomic ions, such as writing symbols and subscripts to balance charges and using a chart to identify polyatomic ion names in compound names.










