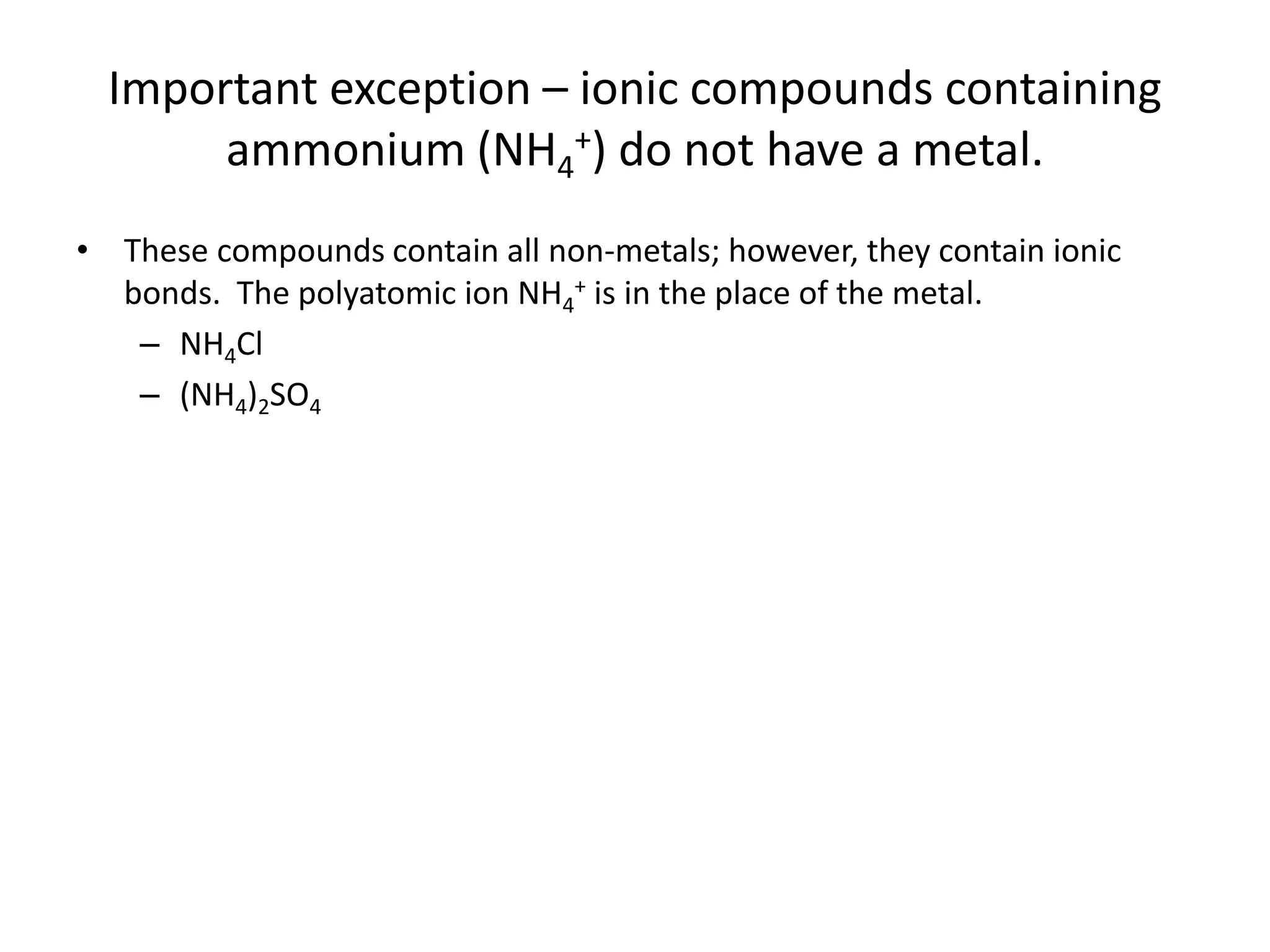
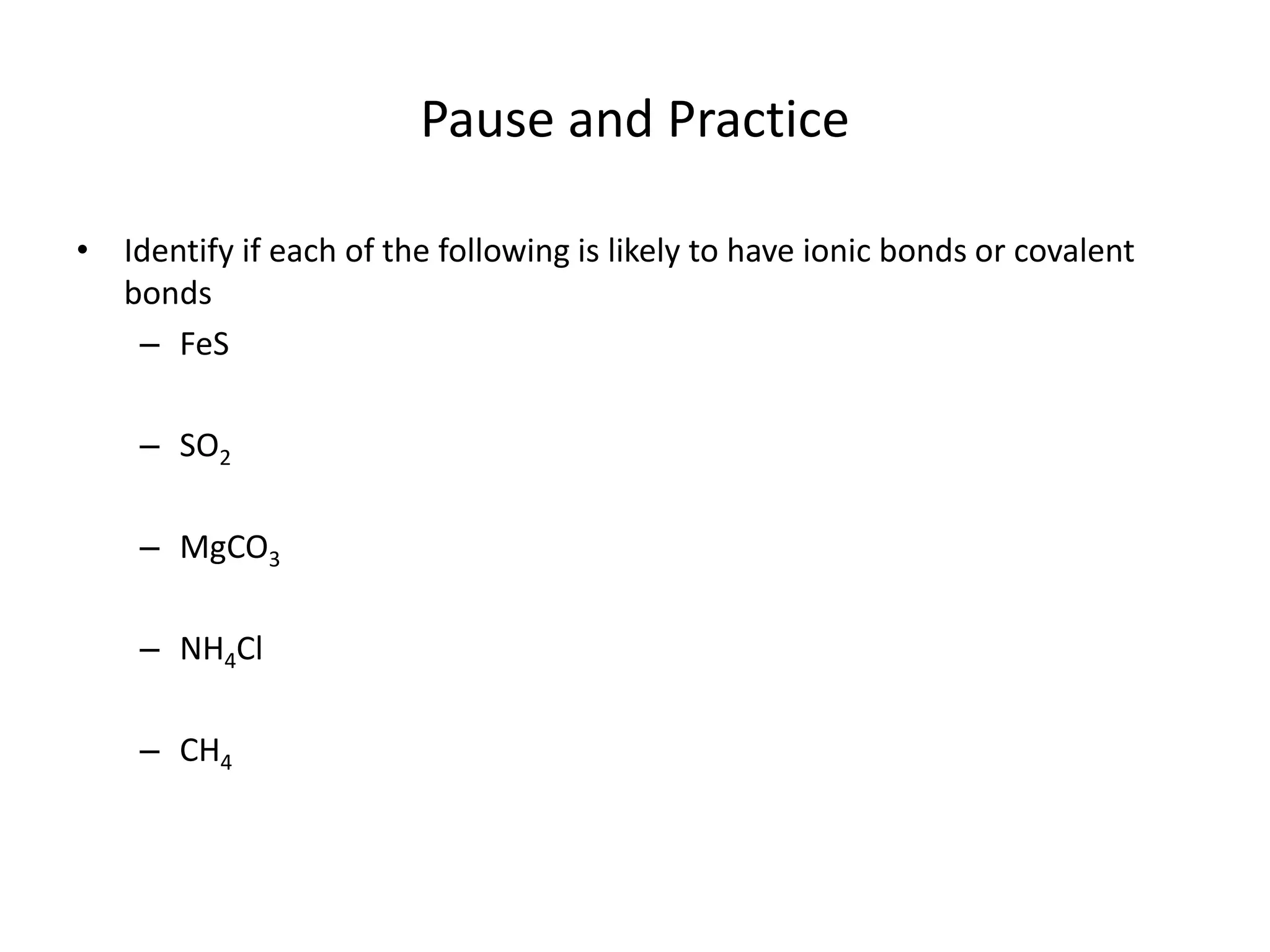
This document discusses how to identify if a substance contains ionic or covalent bonds. It states that if a substance contains a metal and one or more non-metals, it likely contains ionic bonds. If a substance contains all non-metals, it likely contains covalent bonds, with the exception of ammonium compounds which contain ionic bonds despite having no metals. The document provides examples to illustrate these rules and includes a practice problem for the reader.