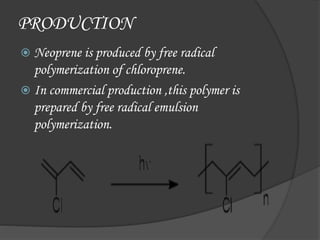
Neoprene is a synthetic rubber produced by polymerizing chloroprene. It exhibits good chemical stability and flexibility over a wide temperature range. It is used in many applications such as laptop sleeves, orthopedic braces, electrical insulation, and automotive fan belts. Neoprene is produced via free radical emulsion polymerization of chloroprene, which is initiated using potassium persulfate and can be produced as either closed cell or open cell forms. It was invented in 1930 by DuPont scientists and is used today due to its resistance to degradation, making it suitable for applications like gaskets, hoses, and corrosion-resistant coatings.




![HISTORY
After DuPont purchased the patent rights
from the university, Wallace Carothers of
DuPont took over commercial development of
Nieuwland's discovery in collaboration with
Nieuwland himself.
Arnold Collins at DuPont focused on mono
vinyl acetylene and reacted the substance
with hydrogen chloride gas,
manufacturing chloroprene.[5]](https://image.slidesharecdn.com/neoprene-170204121534/85/Neoprene-6-320.jpg)



