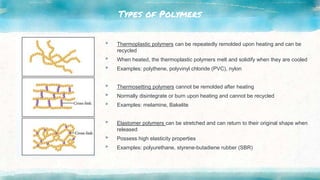
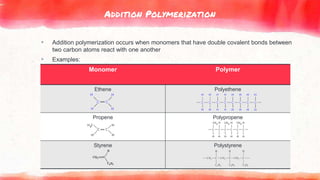
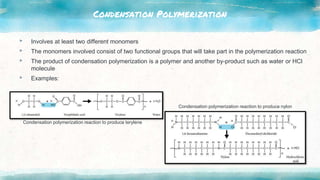
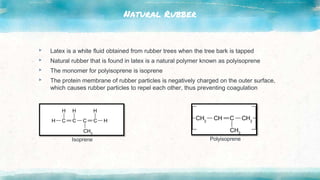
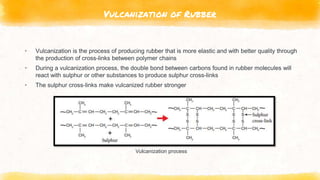
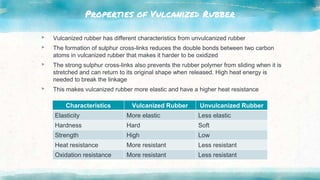
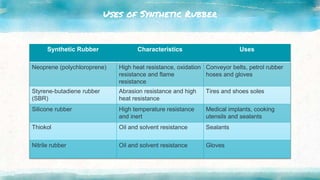
This document discusses polymers, including natural and synthetic polymers. It describes different types of polymers like thermoplastics, thermosets, and elastomers. It also summarizes the two main polymerization reactions - addition polymerization and condensation polymerization. A significant portion of the document focuses on natural rubber, including how it is obtained from rubber trees, its monomer and polymer, and how latex coagulation and vulcanization processes work. Synthetic rubbers are also covered, highlighting examples like neoprene and their uses. The document concludes with notes on the environmental impact of rubber materials.