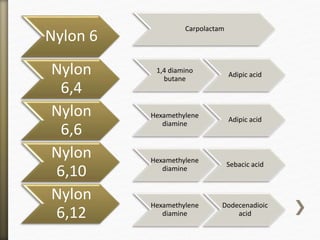
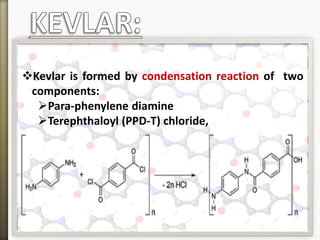
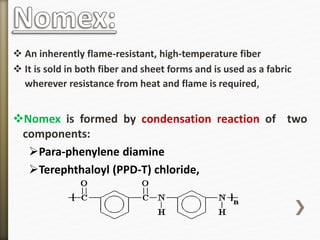
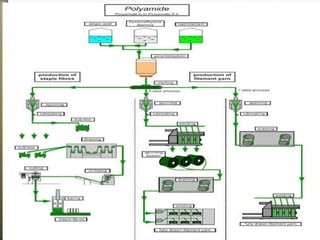
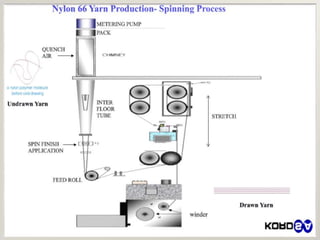
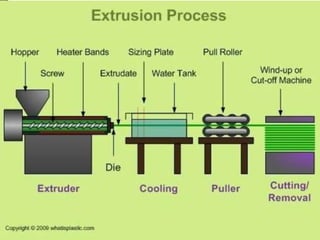
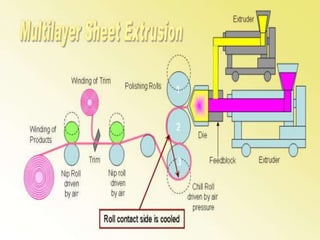
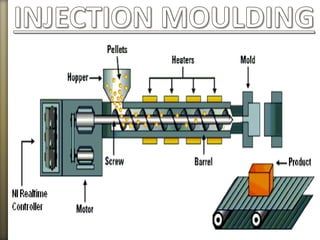
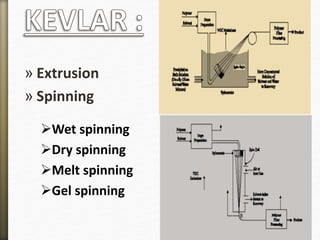
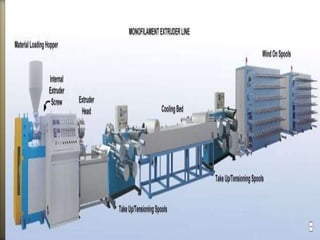
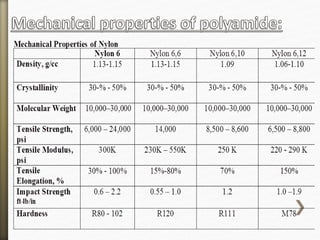
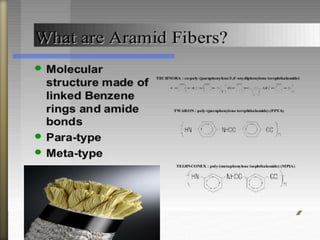
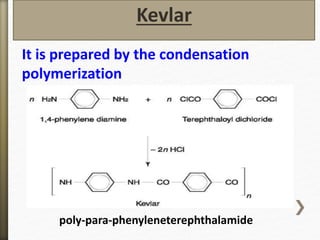
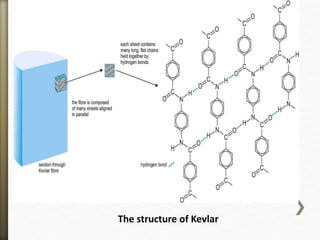
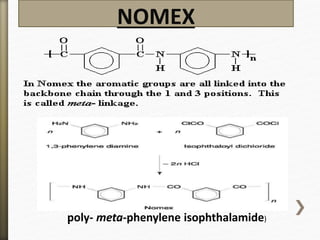
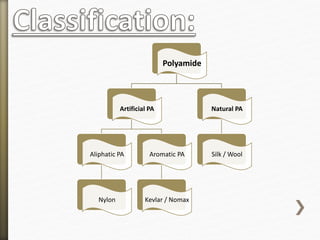
This document discusses polyamides, a polymer family that includes types such as nylon, kevlar, and nomex, detailing their chemical structures, formation processes, and properties. It highlights nylon's development since the 1930s, kevlar's strength and flame resistance, and nomex's applications in high-temperature environments. The document also outlines manufacturing methods for polyamides and their various industrial and household applications.






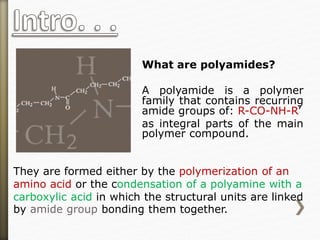

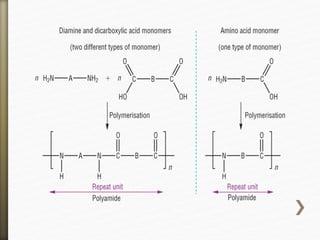
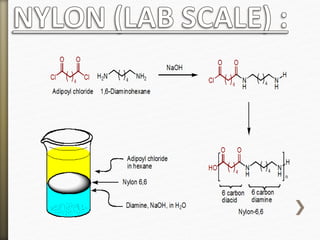
![Nylon is formed by the condensation reaction of
two components:
Diamine (a compound containing two amino
[NH2] groups—e.g., hexamethylenediamine)
Dicarboxylic acid (containing two carboxyl
[CO−OH] groups—e.g., adipic acid),
Or may be formed by the self-condensation of an
amino acid or an amino-acid derivative.](https://image.slidesharecdn.com/20-11-141218115404-conversion-gate02/85/polyamides-11-320.jpg)