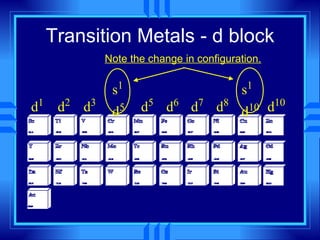
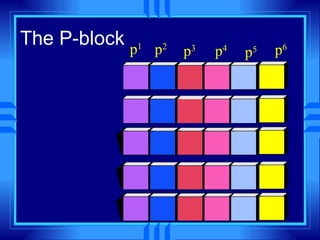
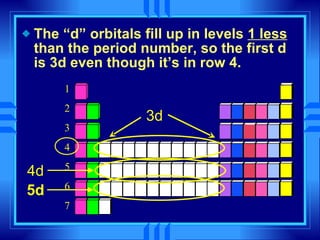
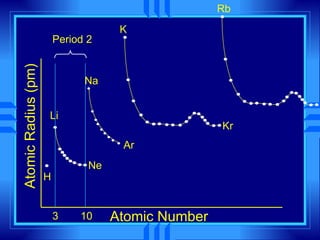
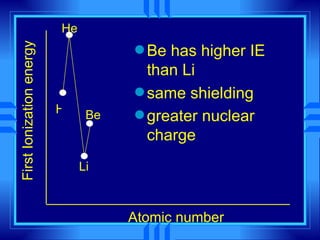
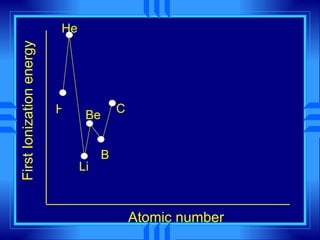
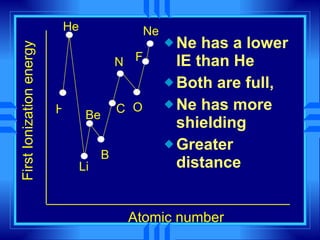
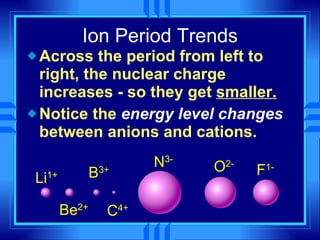
This document summarizes key concepts from a chemistry textbook chapter on the periodic table. It discusses how elements are organized in the periodic table based on their atomic structure and properties. Early sections describe the historical development of periodic tables and how elements are classified into groups based on electron configuration. Later sections summarize periodic trends in atomic size, ionization energy, and ion size based on an element's position in the periodic table and how its electron configuration is filled.