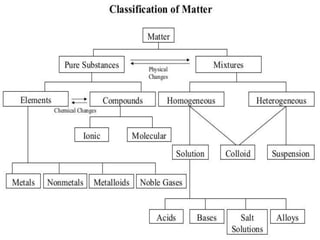
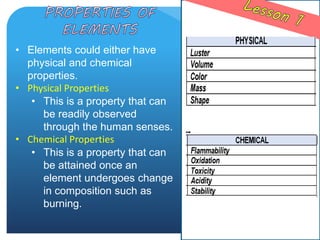
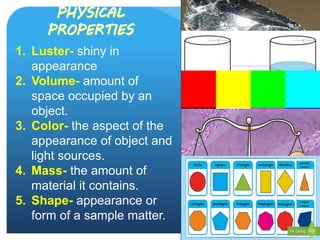
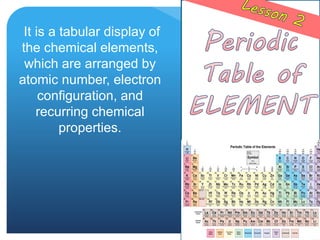
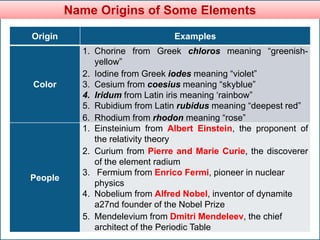
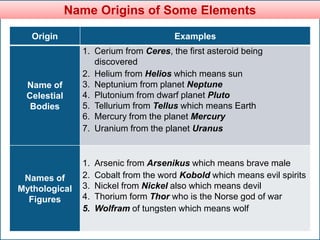
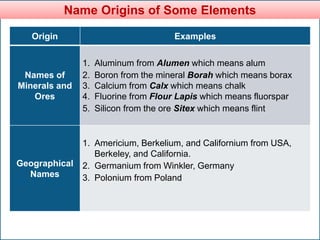
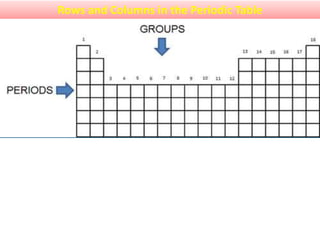
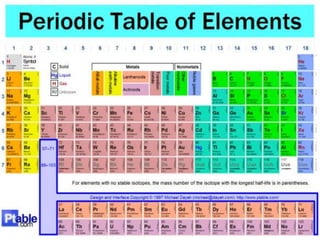
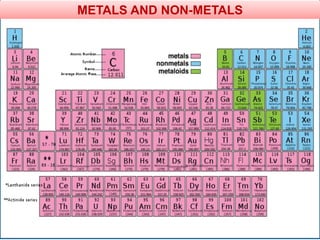
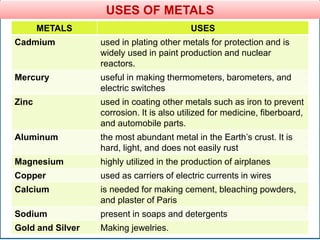
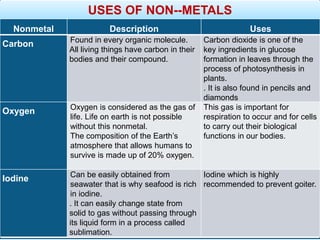
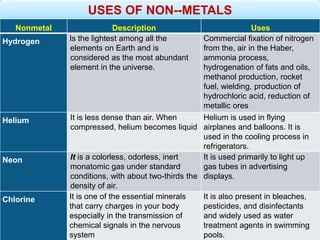
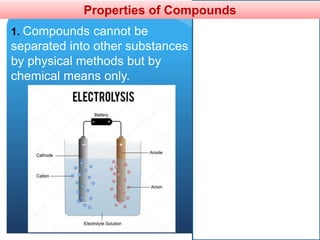
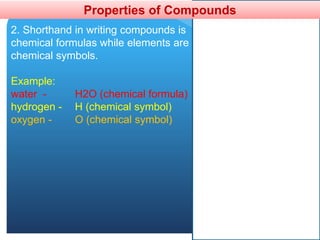
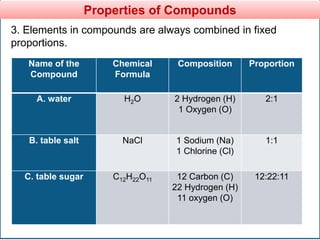
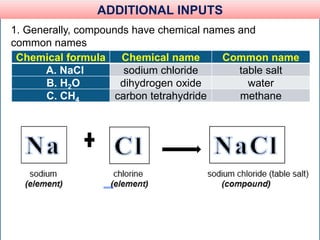
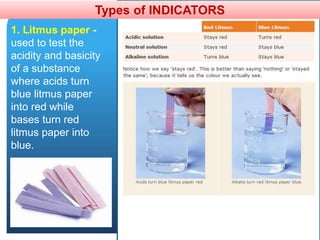
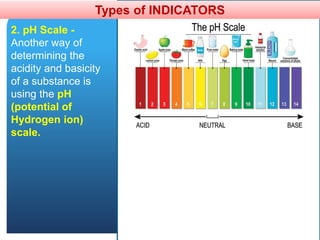
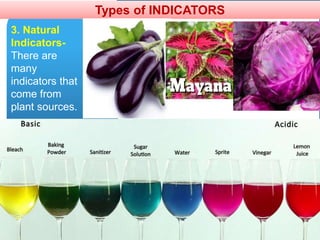
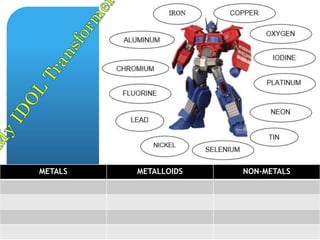
The document provides information about elements and compounds for 7th grade science. It begins by outlining the objectives of describing elements and compounds, explaining the difference between them, and citing examples. It then defines matter and discusses the three states of matter. It explains that elements are pure substances that cannot be broken down further, while compounds are formed by combining two or more elements. The document provides numerous examples of elements and compounds. It also discusses the periodic table and properties of metals, nonmetals and metalloids.