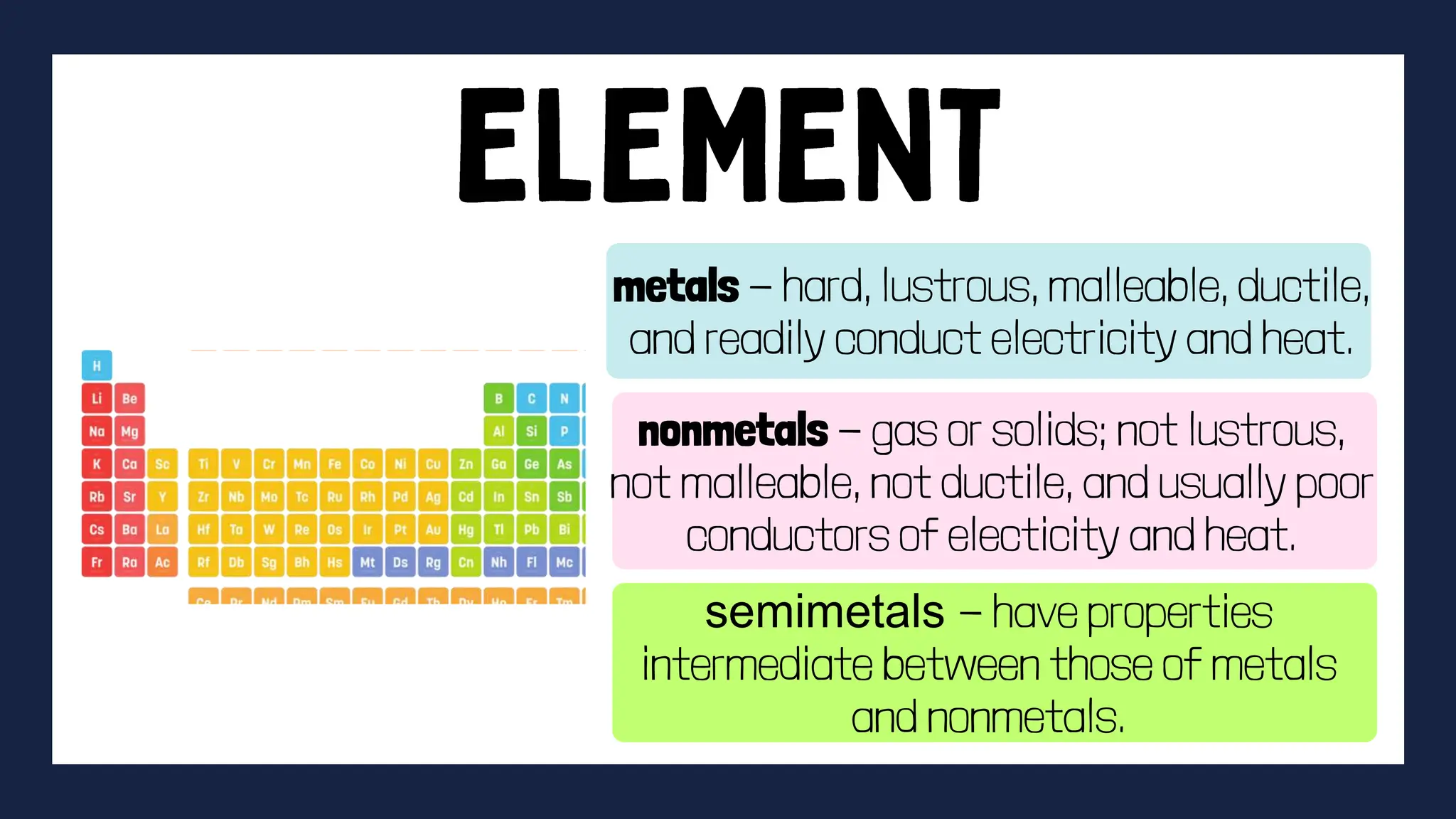
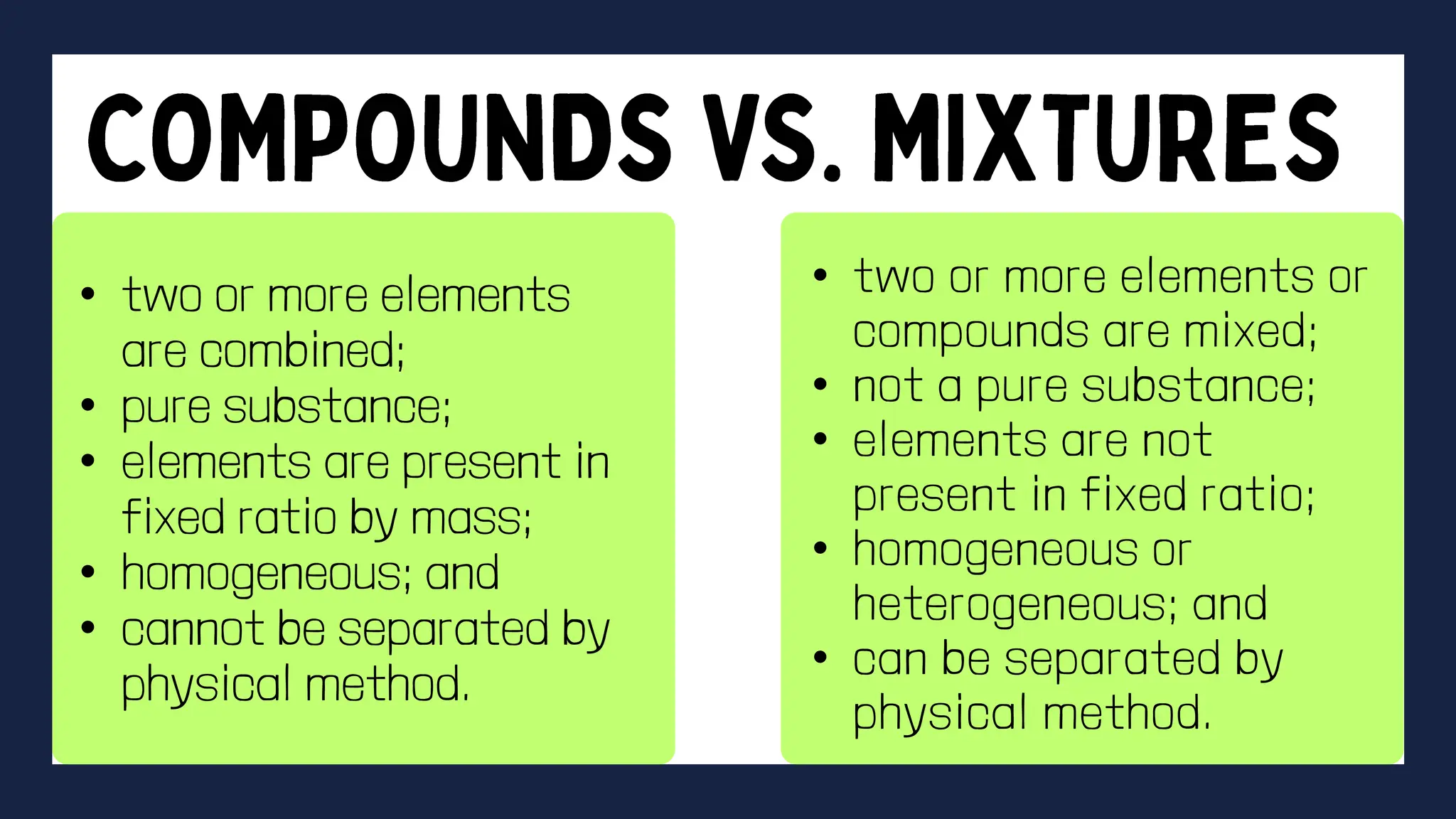
This document discusses the differences between elements and compounds. It defines elements as substances made of only one type of atom that cannot be broken down further by chemical means. Compounds are made of two or more elements and can be broken down into simpler substances through chemical reactions. The document provides examples of common elements and compounds and classifies elements as metals, nonmetals and semimetals based on their physical properties. It also gives guidelines for an activity asking students to identify examples of elements and compounds found at home and discuss their importance.