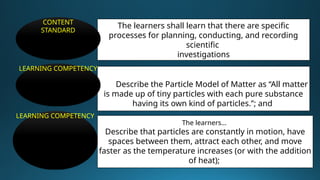
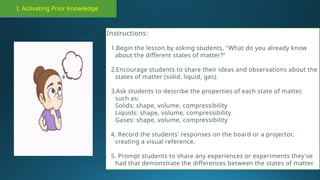
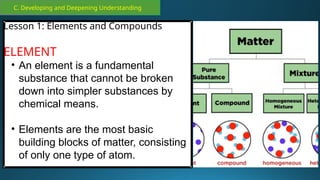
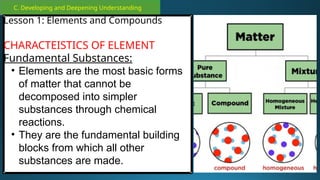
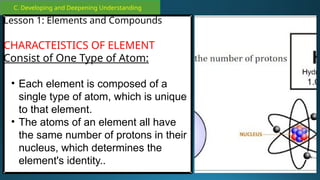
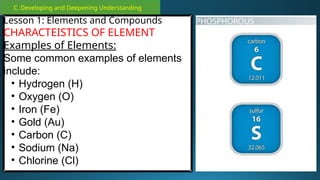
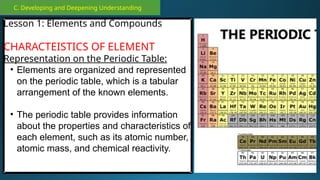
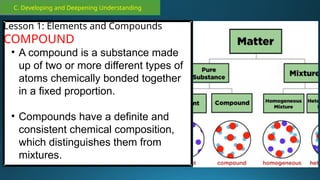
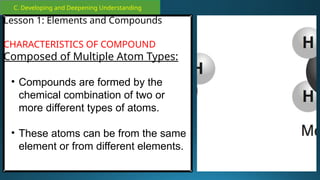
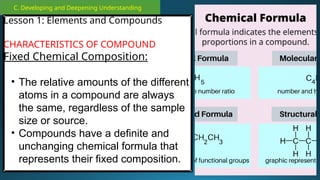
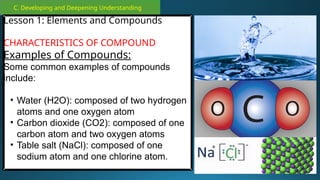
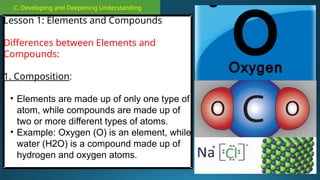
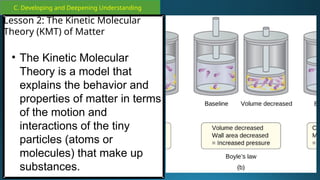
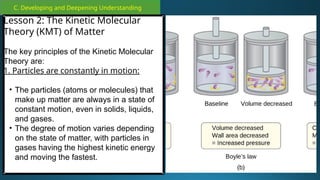
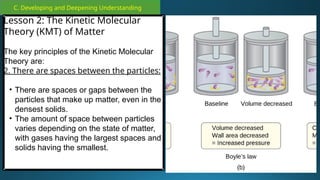
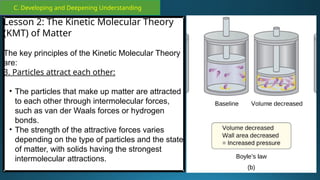
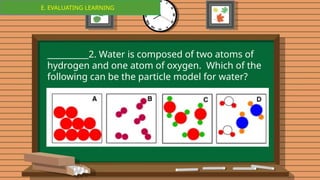
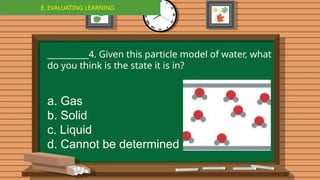
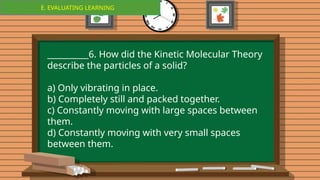
The document outlines a lesson plan focused on the kinetic molecular theory (KMT) and the distinction between elements and compounds, aiming for students to grasp core concepts related to matter's behavior. Key learning objectives include defining 'pure substance,' appreciating KMT, and conducting experiments to observe particle behavior. The plan emphasizes active participation through discussions, hands-on activities, and reflections to deepen understanding of molecular motion and properties.