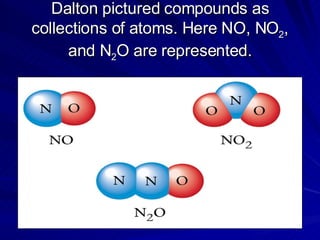
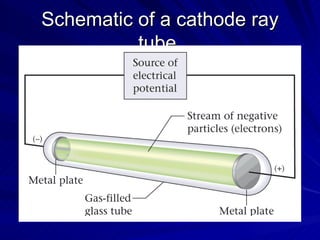
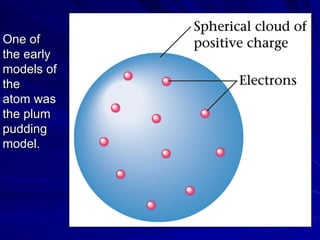
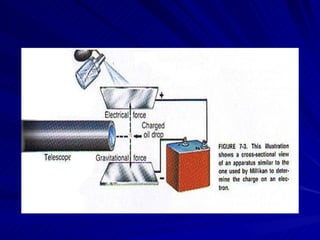
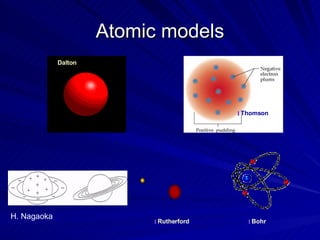
1. Early atomic models proposed by philosophers like Democritus and Dalton proposed that atoms were the fundamental indivisible units of matter.
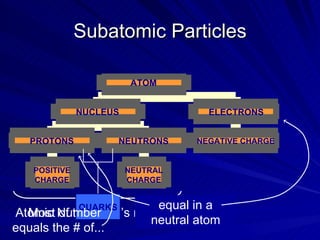
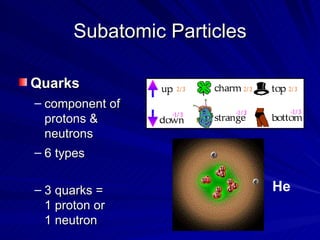
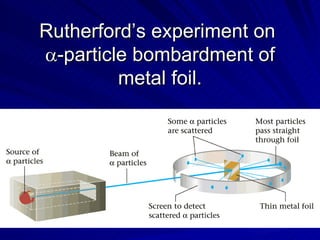
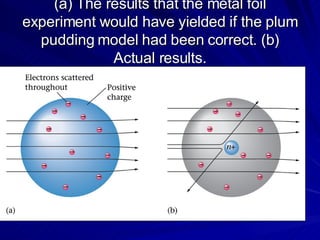
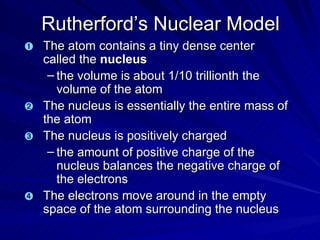
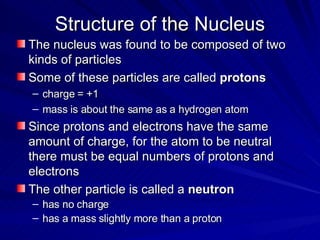
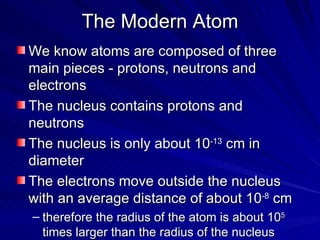
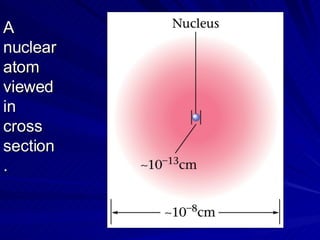
2. Rutherford's gold foil experiment in the early 1900s showed that the atom has a small, dense nucleus at its center containing positive charge.
3. Later models like Bohr's incorporated the idea that electrons orbit the nucleus in fixed energy levels, accounting for the emission and absorption of photons.