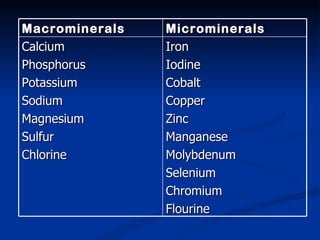
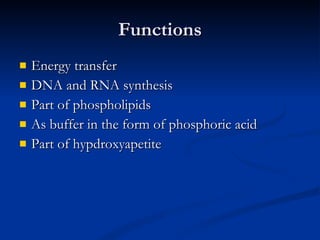
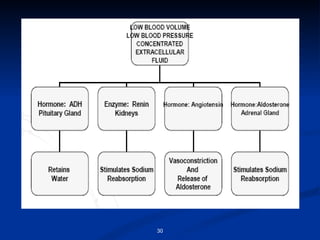
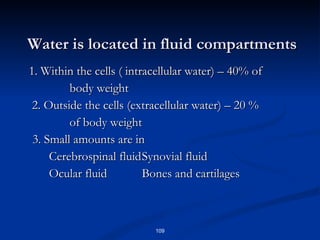
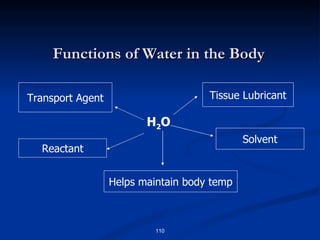
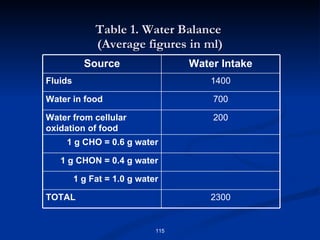
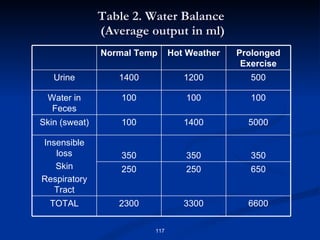
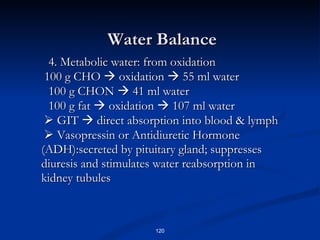
Minerals play important structural and regulatory roles in the body. Major minerals, also called macrominerals, include calcium, phosphorus, magnesium, and sodium, which are present in larger amounts and are crucial for bones, teeth, muscle and nerve function. Trace minerals, also called microminerals, such as iron, zinc, selenium, and manganese, are needed in smaller amounts but still essential as components of enzymes and for other metabolic processes. Minerals are obtained from foods and their absorption and balance in the body are tightly controlled. Deficiencies or toxicities can occur if intake is inadequate or excessive.