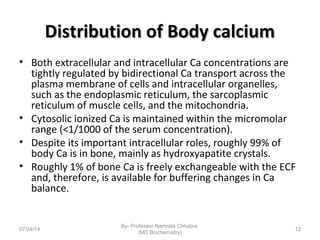
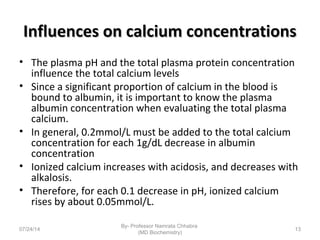
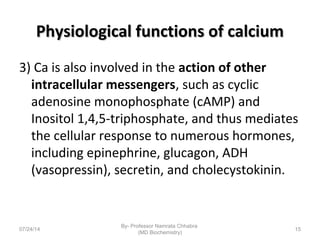
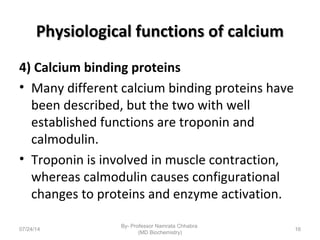
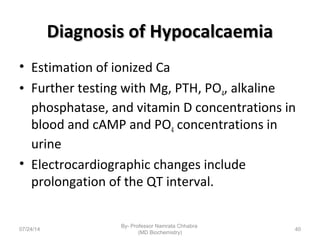
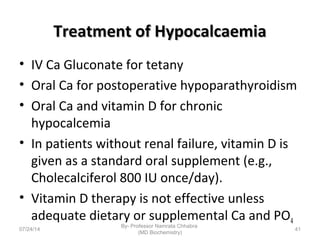
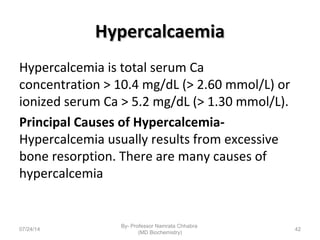
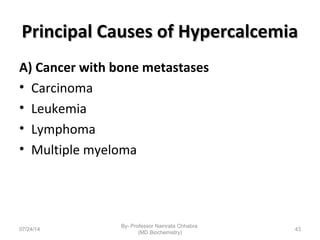
1) Calcium is essential for muscle contraction, nerve conduction, hormone release, and blood coagulation. The daily intake is approximately 1000mg, found in foods like milk, cheese, fish, and beans.
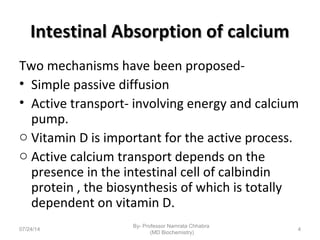
2) Calcium is absorbed in the small intestine through both passive diffusion and active transport involving vitamin D. Around 30-80% is absorbed depending on dietary intake.
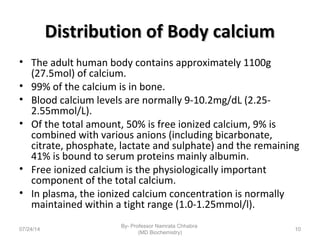
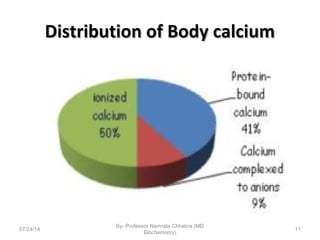
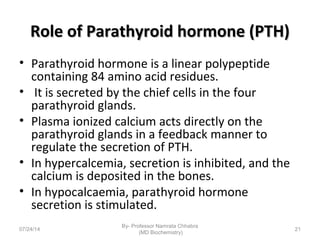
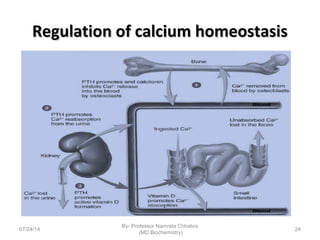
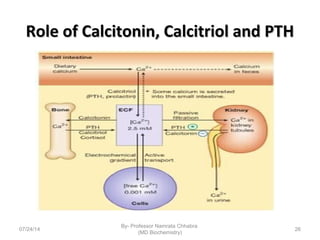
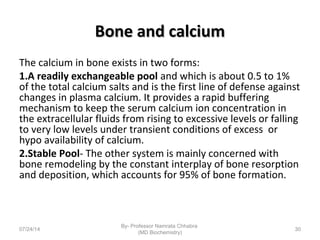
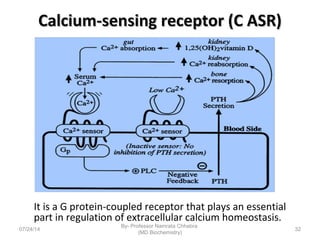
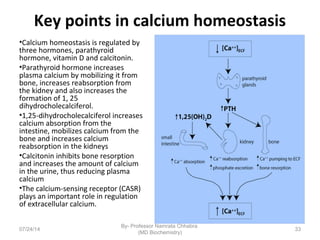
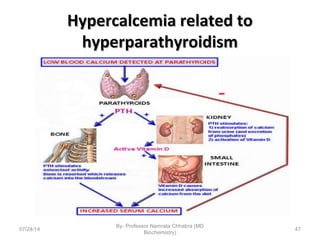
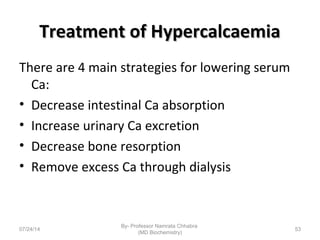
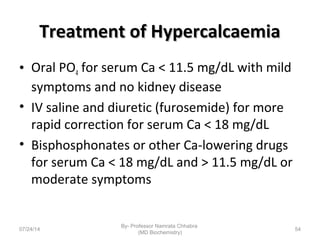
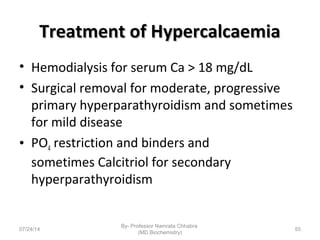
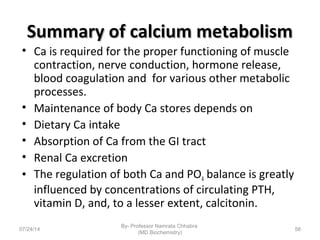
3) Calcium levels in the body are tightly regulated by parathyroid hormone, vitamin D, and calcitonin which act on the intestines, bone, and kidneys to influence absorption, resorption, and excretion.