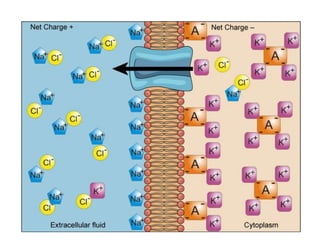
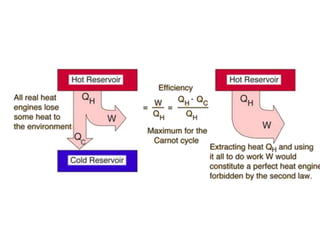
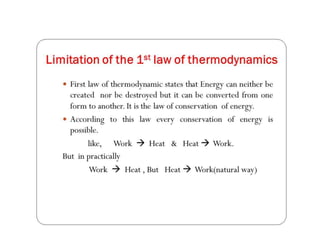
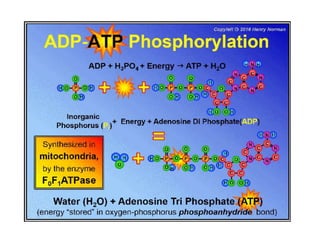
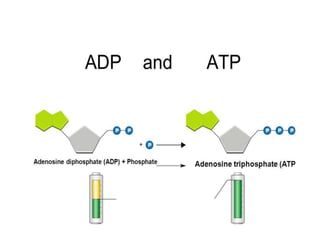
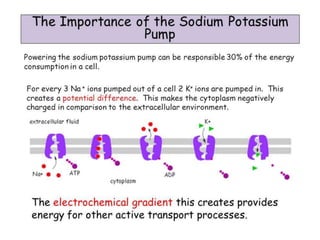
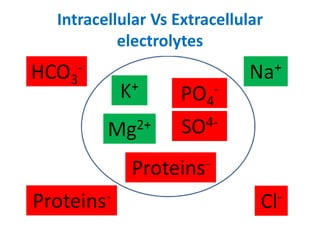
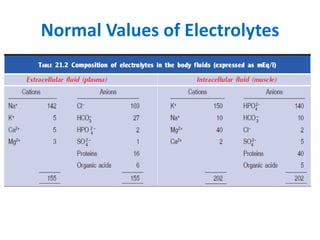
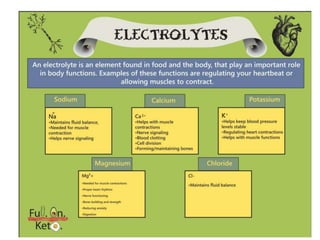
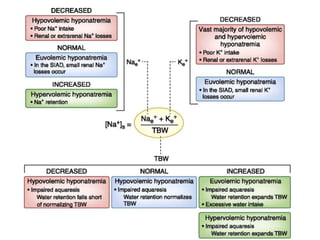
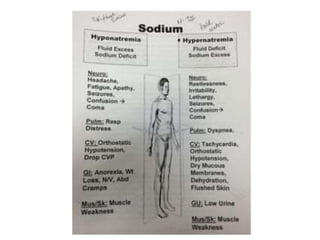
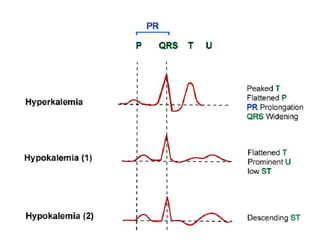
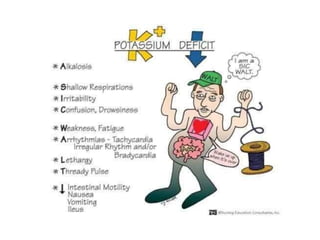
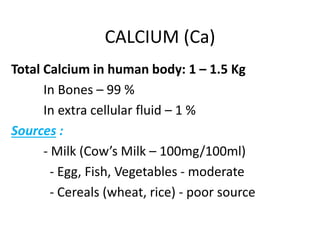
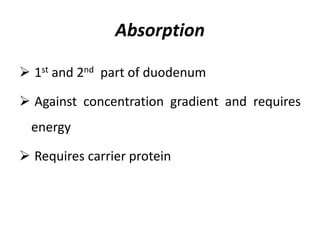
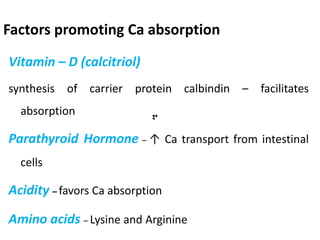
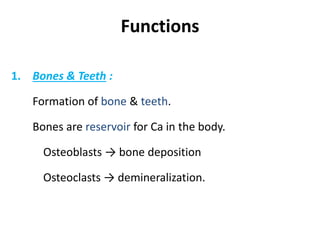
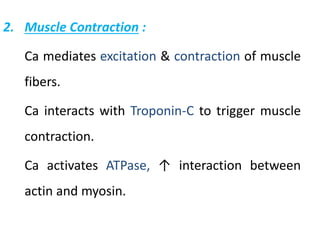
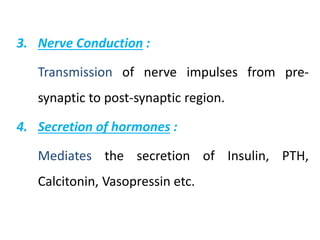
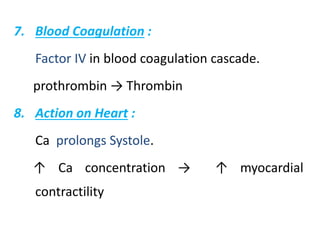
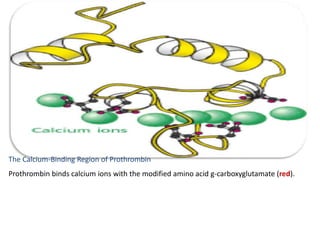
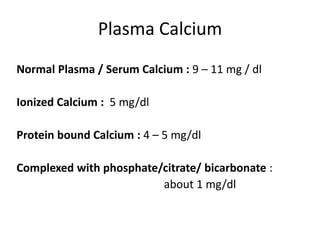
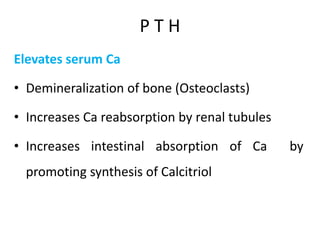
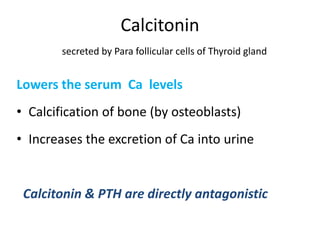
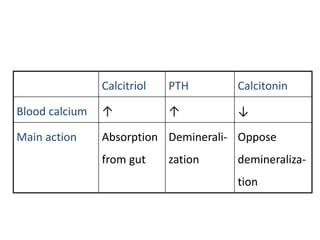
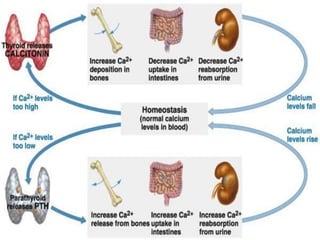
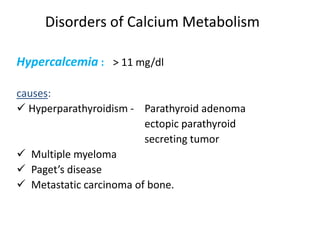
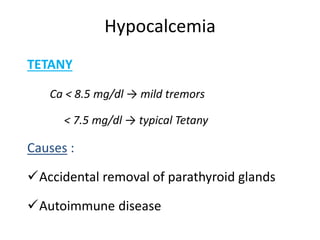
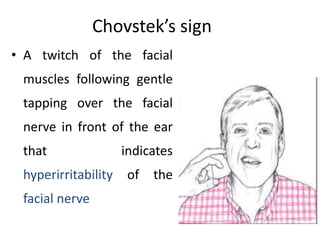
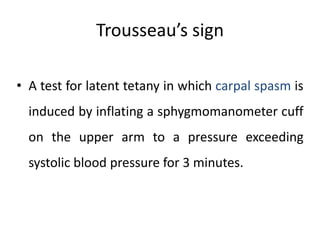
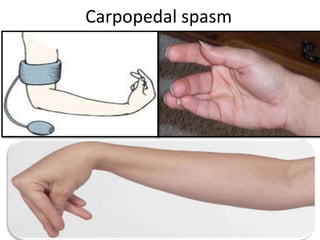
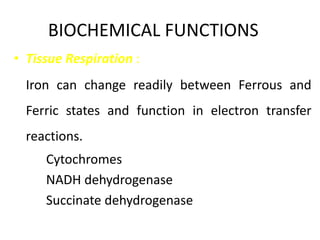
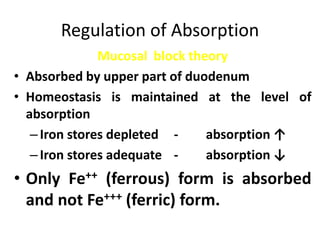
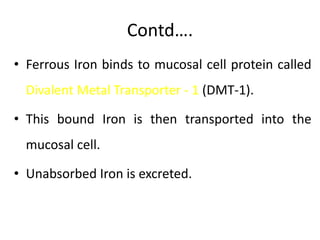
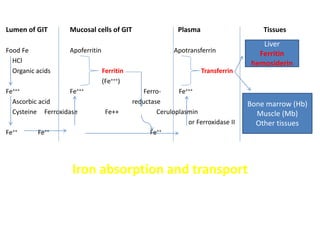
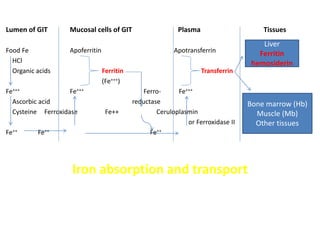
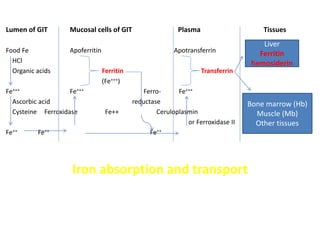
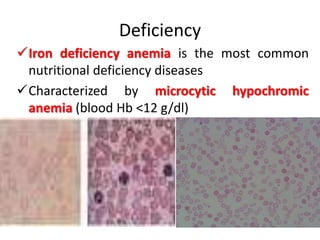
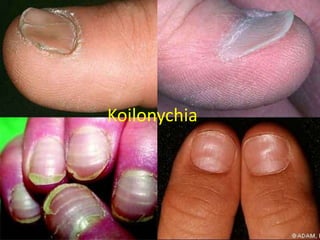
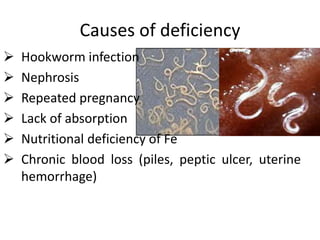
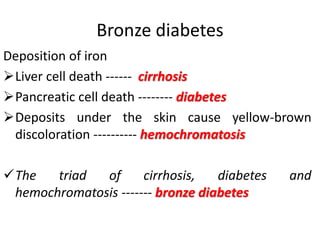
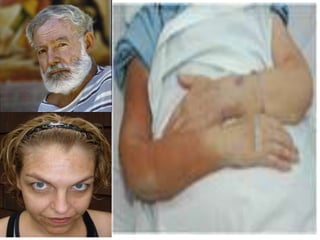
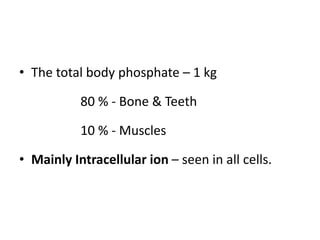
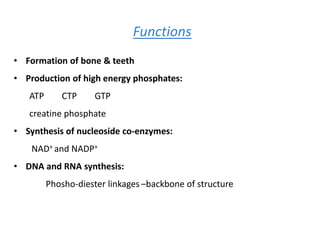
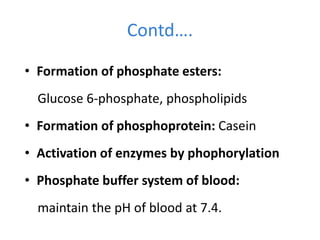
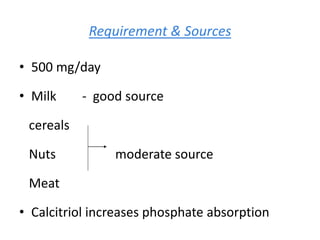
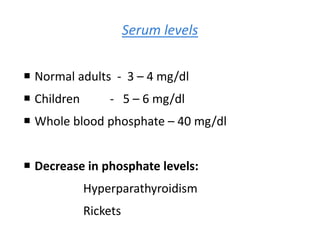
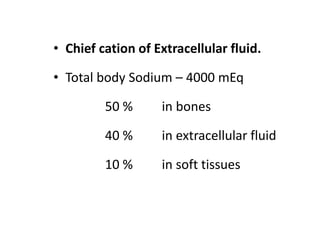
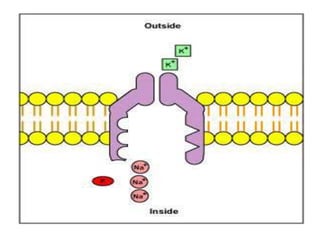
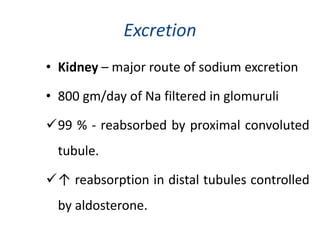
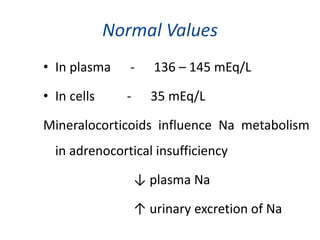
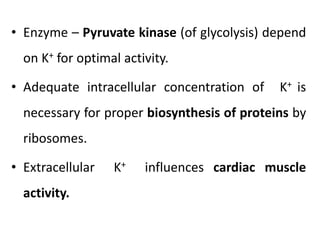
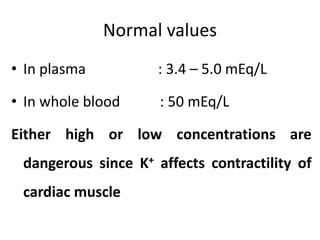
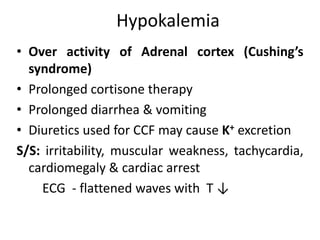
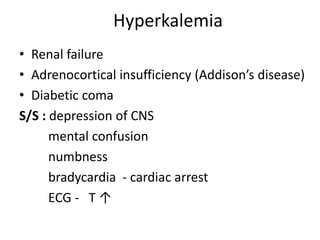
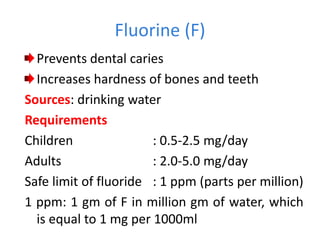
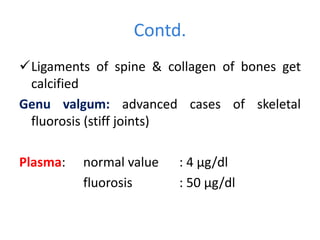
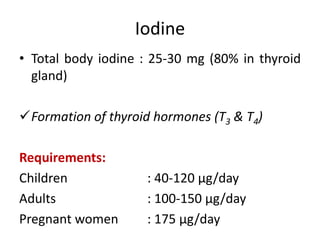
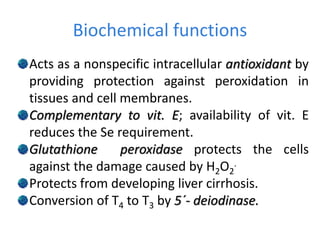
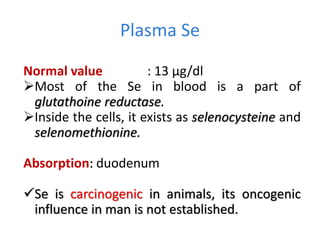
Minerals play essential roles in the human body. This document discusses several important minerals, including calcium and iron. Calcium is critical for bone and teeth formation, muscle contraction, nerve conduction, and blood coagulation. Homeostasis of calcium levels is regulated by calcitriol, parathyroid hormone, and calcitonin. Disorders can result in hypercalcemia or hypocalcemia. Iron is essential for tissue respiration, oxygen transport, and immune function. Iron absorption is regulated by stores in the body and occurs primarily in the duodenum. Iron deficiency is a concern, especially in women, and dietary sources and requirements are discussed.