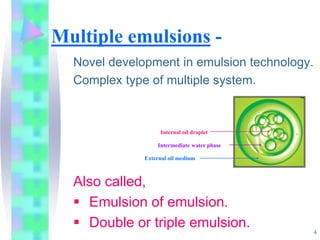
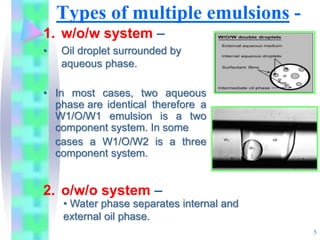
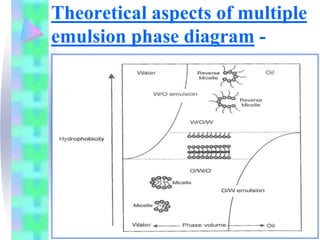
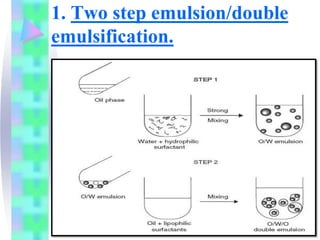
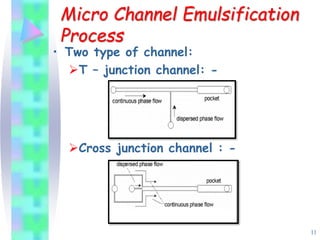
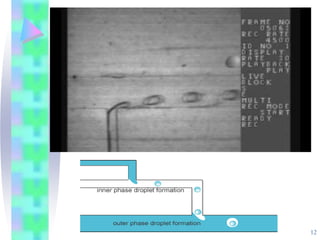
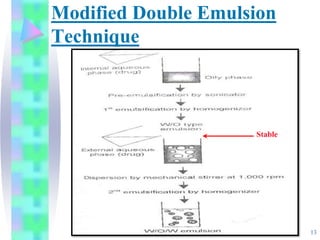
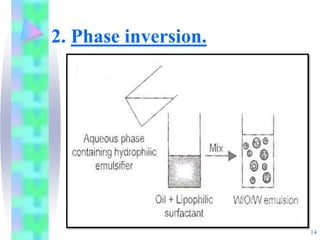
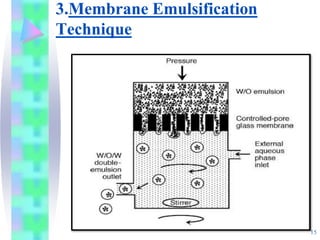
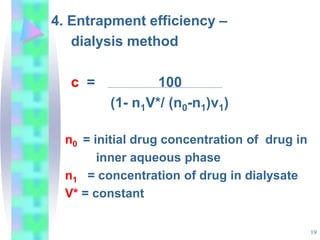
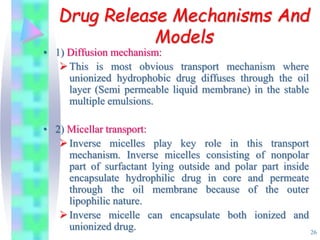
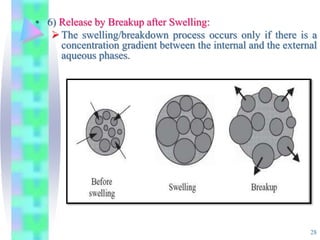
This document discusses multiple emulsions, which are complex emulsion systems containing an internal oil droplet phase surrounded by an intermediate water phase and external oil medium. The key types are w/o/w and o/w/o emulsions. Multiple emulsions offer advantages like protecting actives, high encapsulation, and controlled release. However, they are thermodynamically unstable. The document outlines methods for producing and stabilizing multiple emulsions, including double emulsification and phase inversion techniques. Characterization methods and factors affecting preparation are also summarized. Applications include controlled drug delivery, targeting, and use in cosmetics, food, and oxygen delivery.