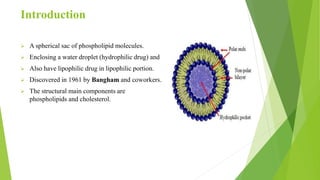
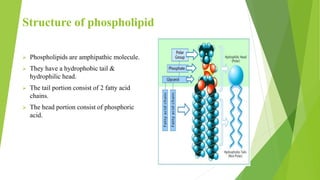
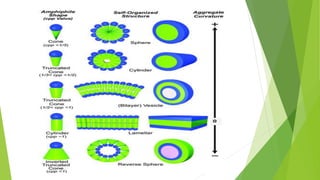
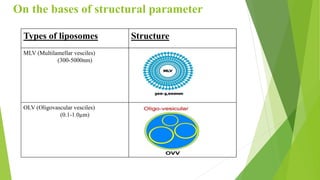
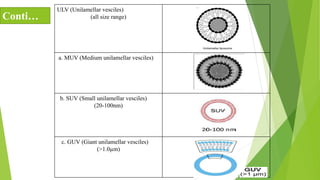
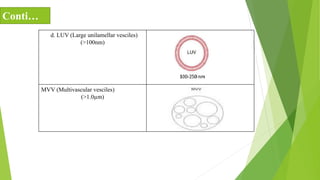
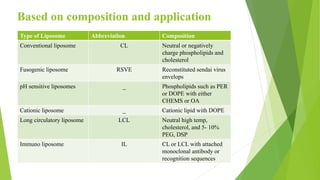
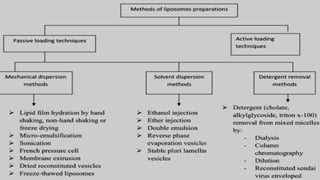
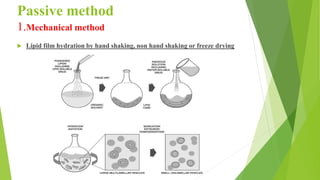
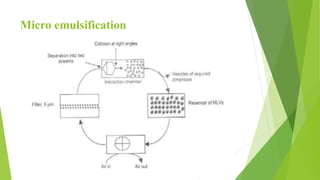
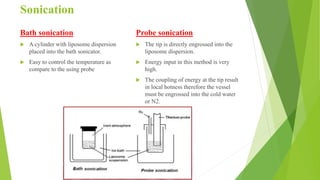
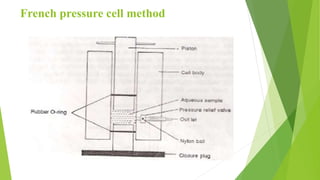
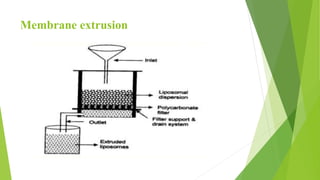
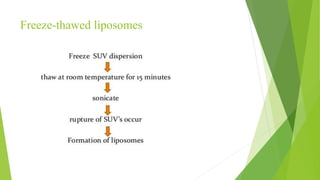
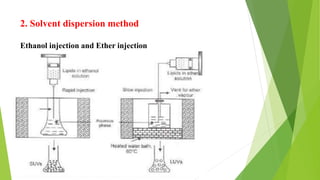
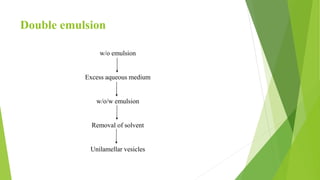
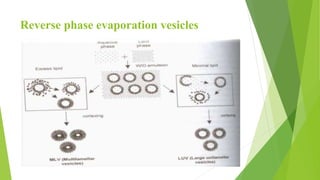
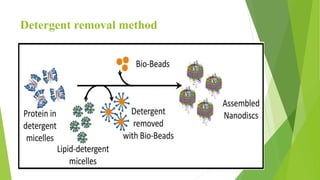
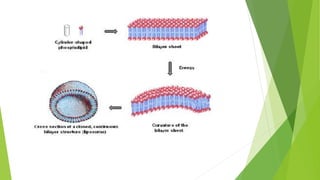
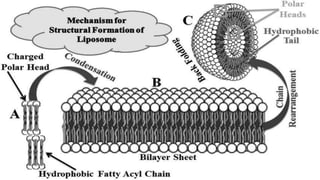
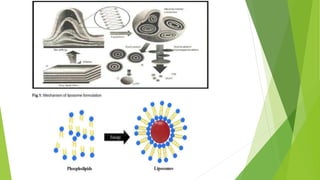
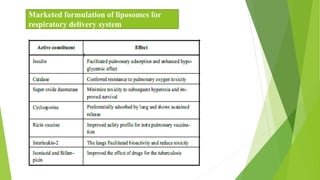
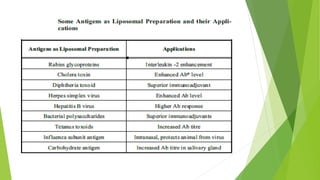
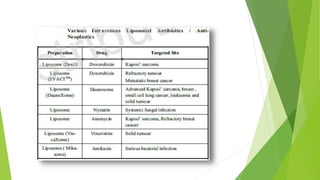
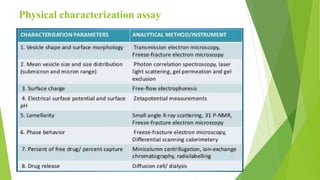
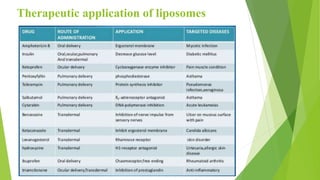
The document discusses liposome preparation and evaluation, covering their structure, advantages, disadvantages, classification, methods of preparation, and therapeutic applications. Liposomes are spherical phospholipid structures that encapsulate drugs, enhancing efficacy and reducing toxicity, yet facing challenges such as instability and high production costs. The document concludes by highlighting their potential in various medical applications and their established role in modern drug delivery systems.