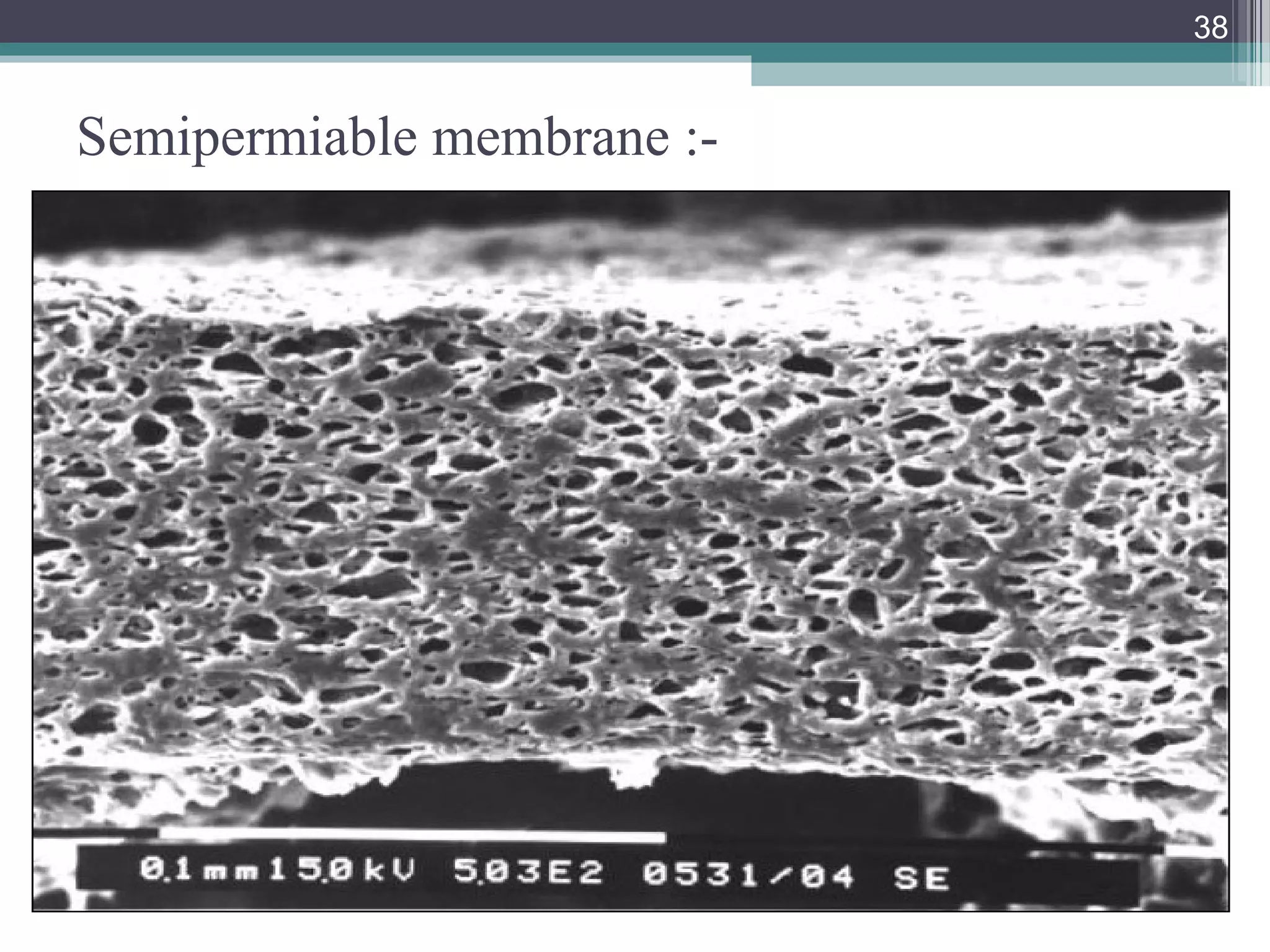
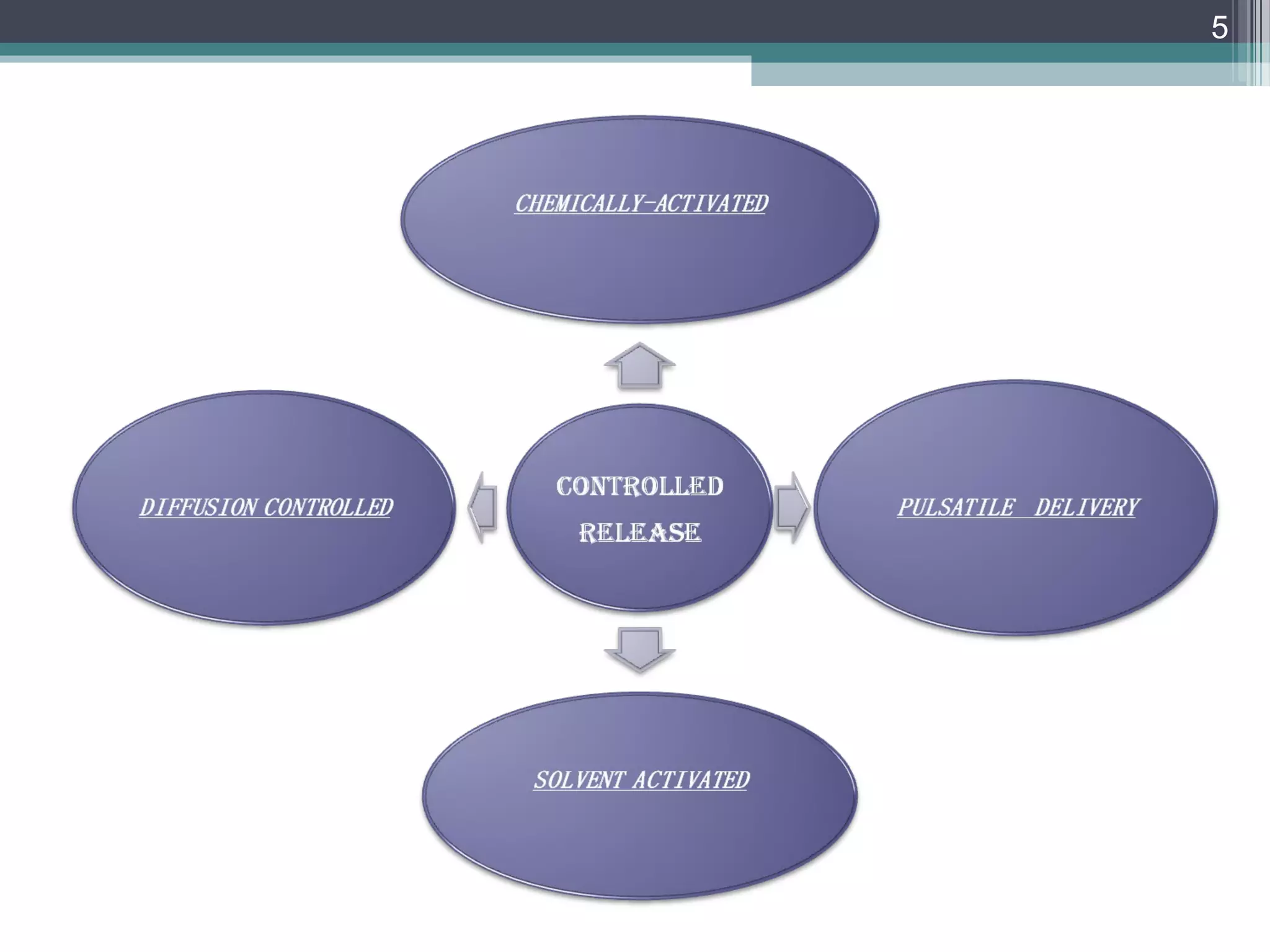
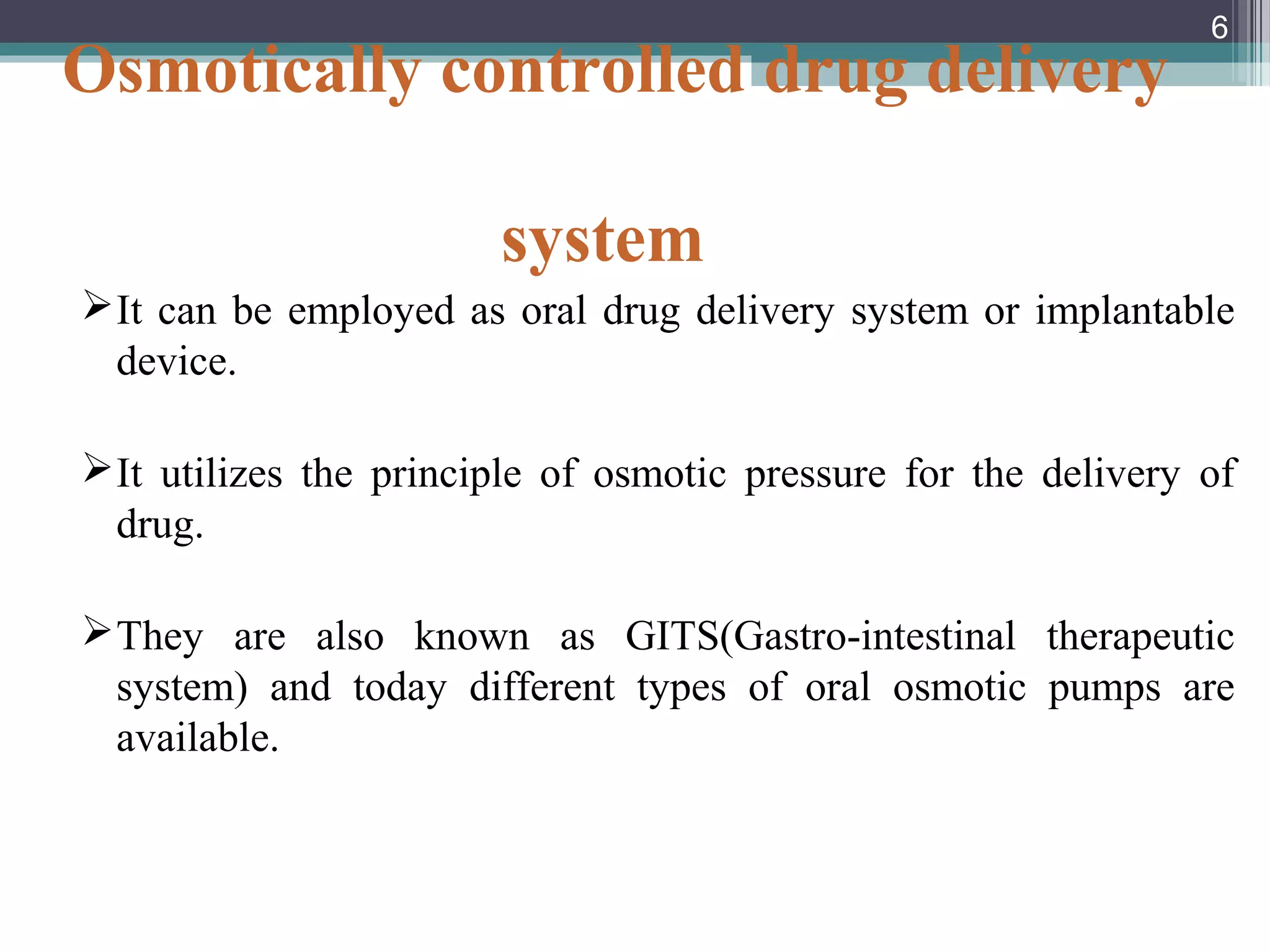
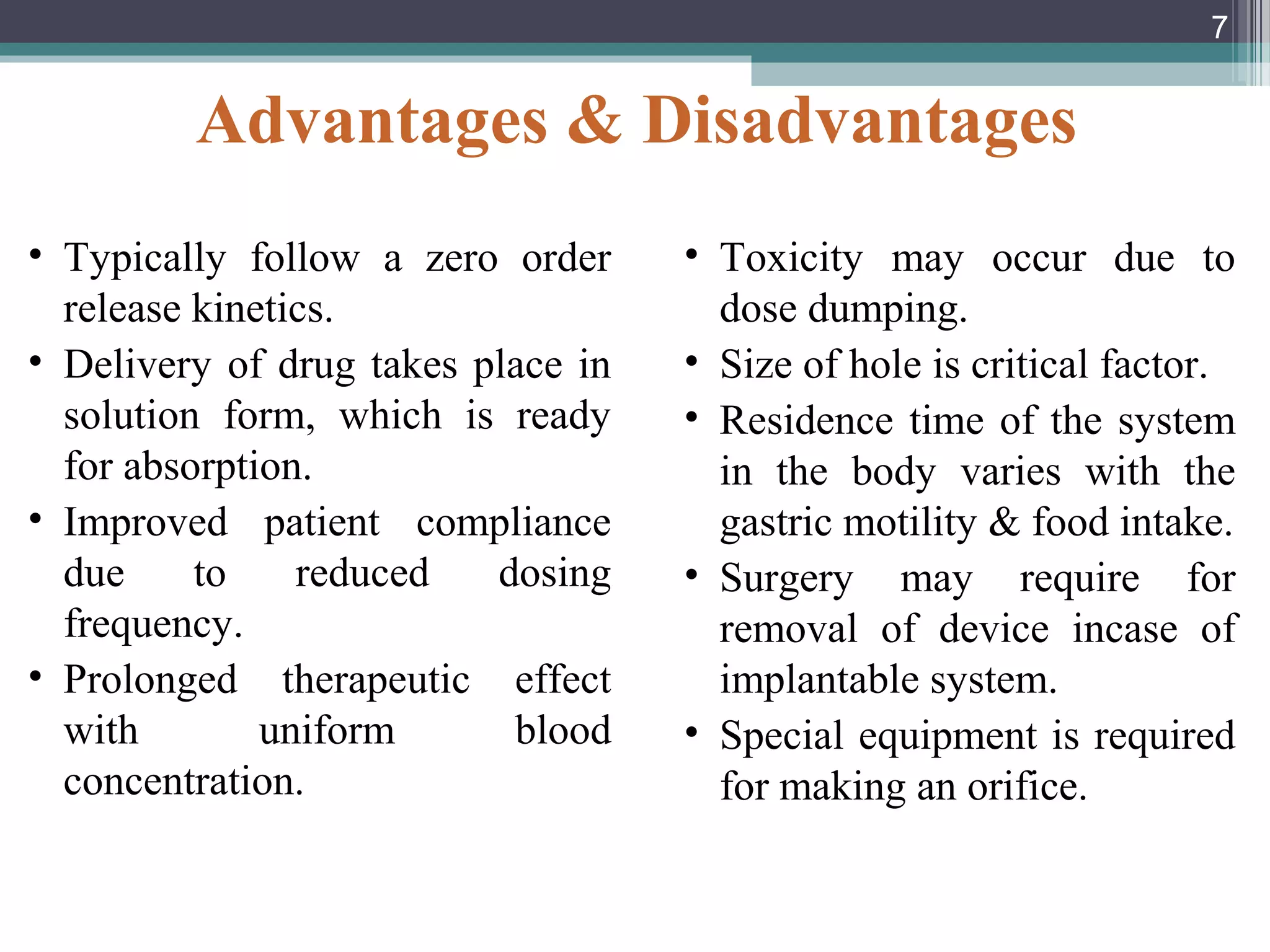
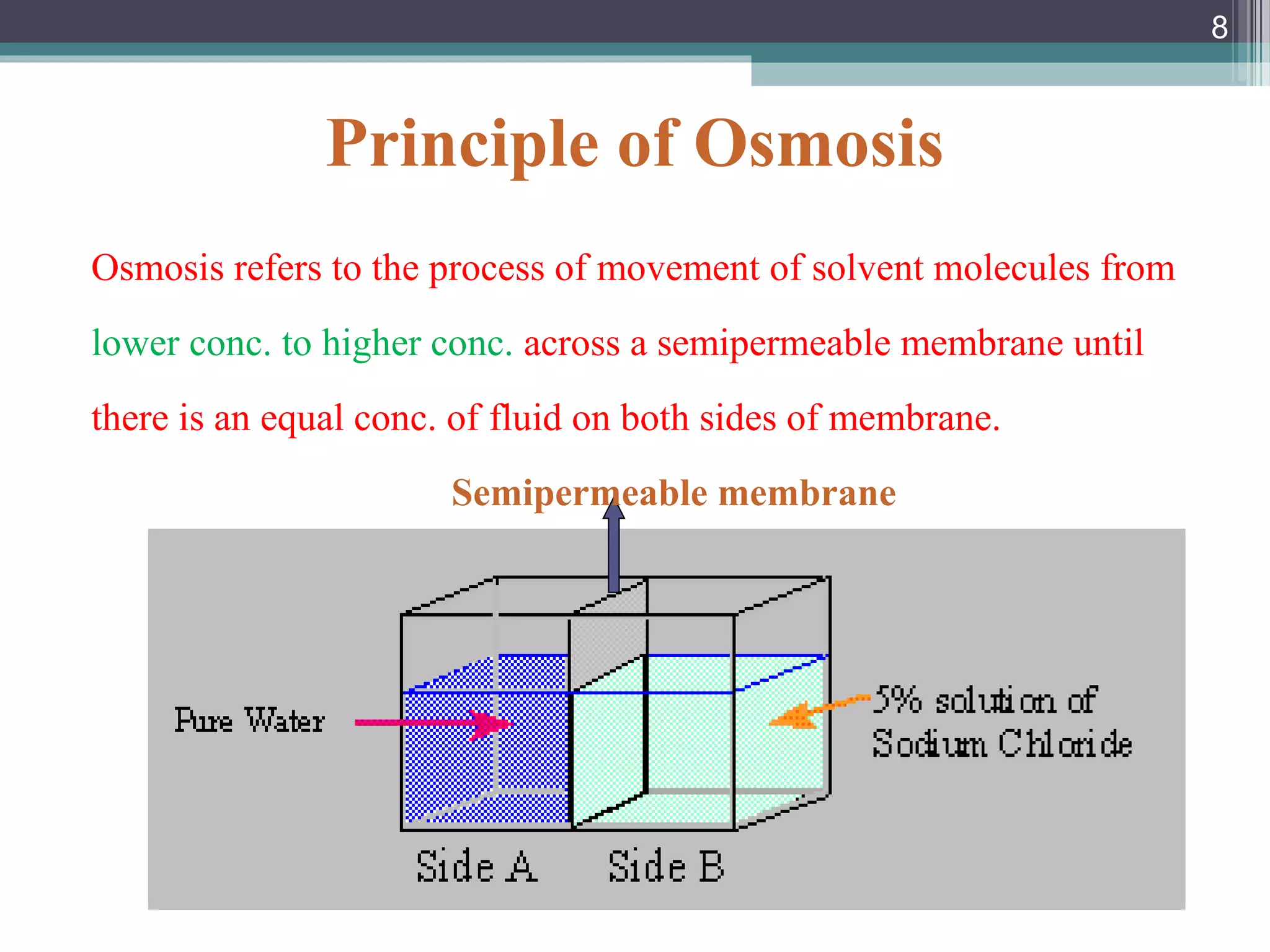
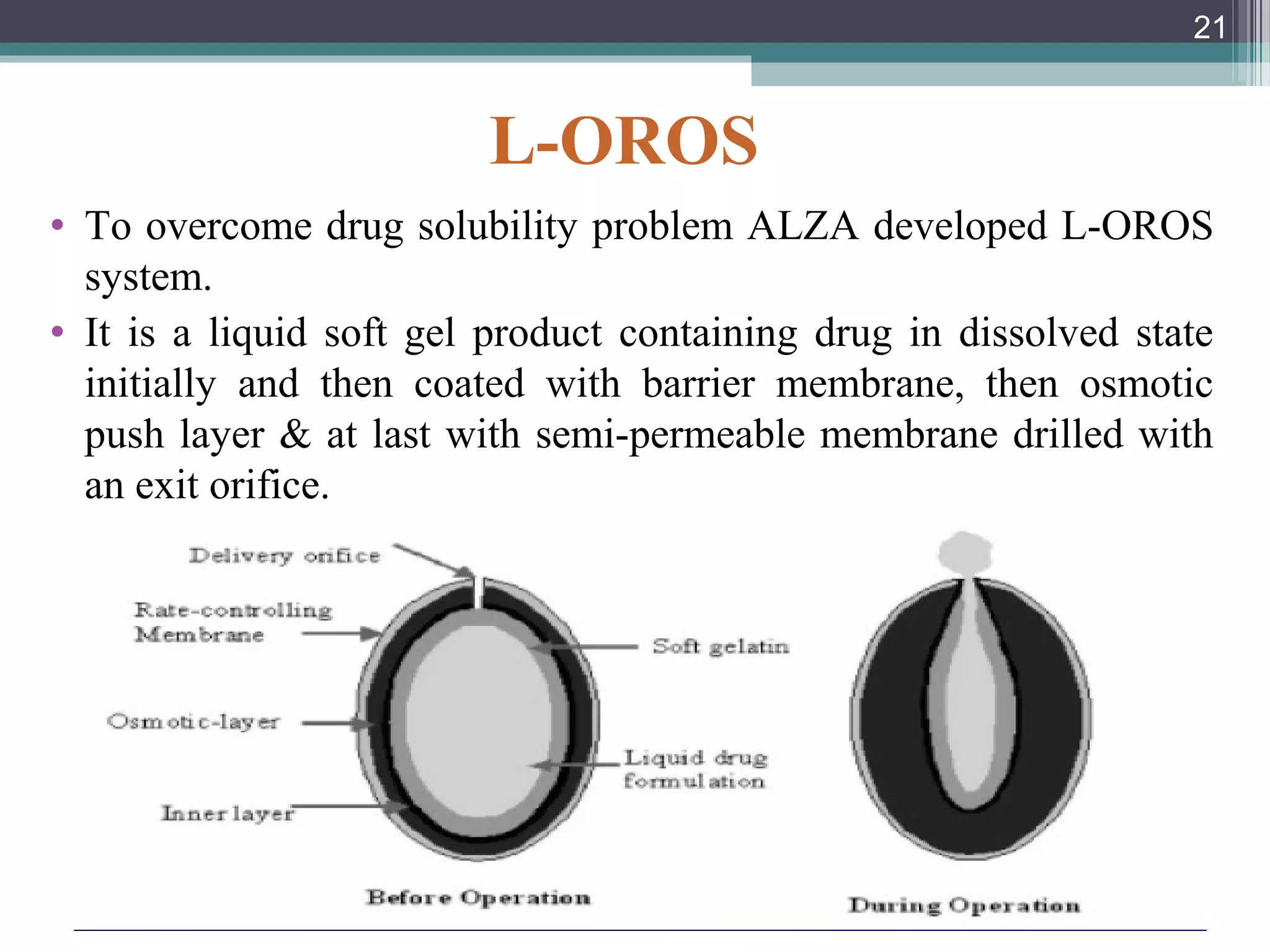
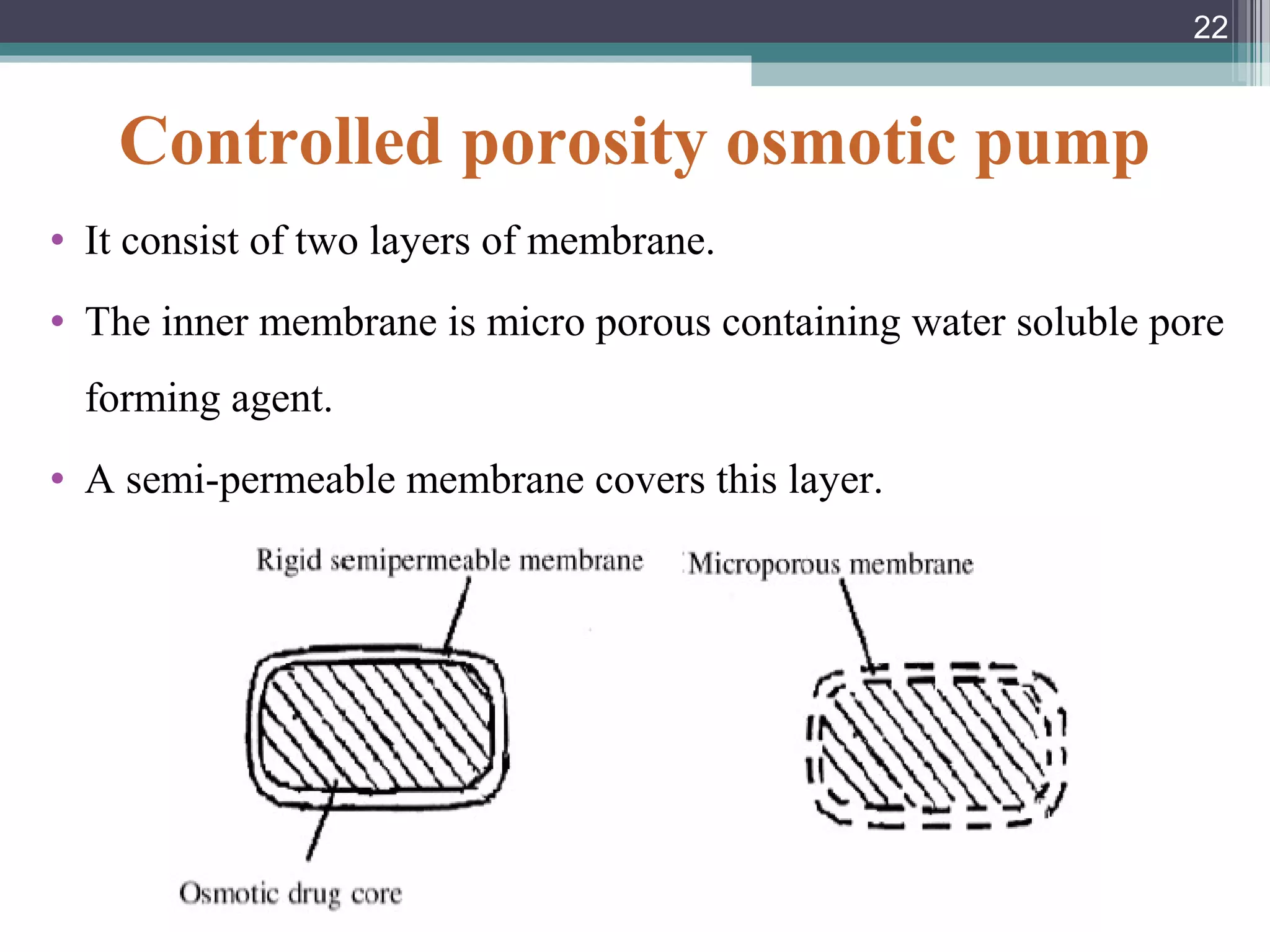
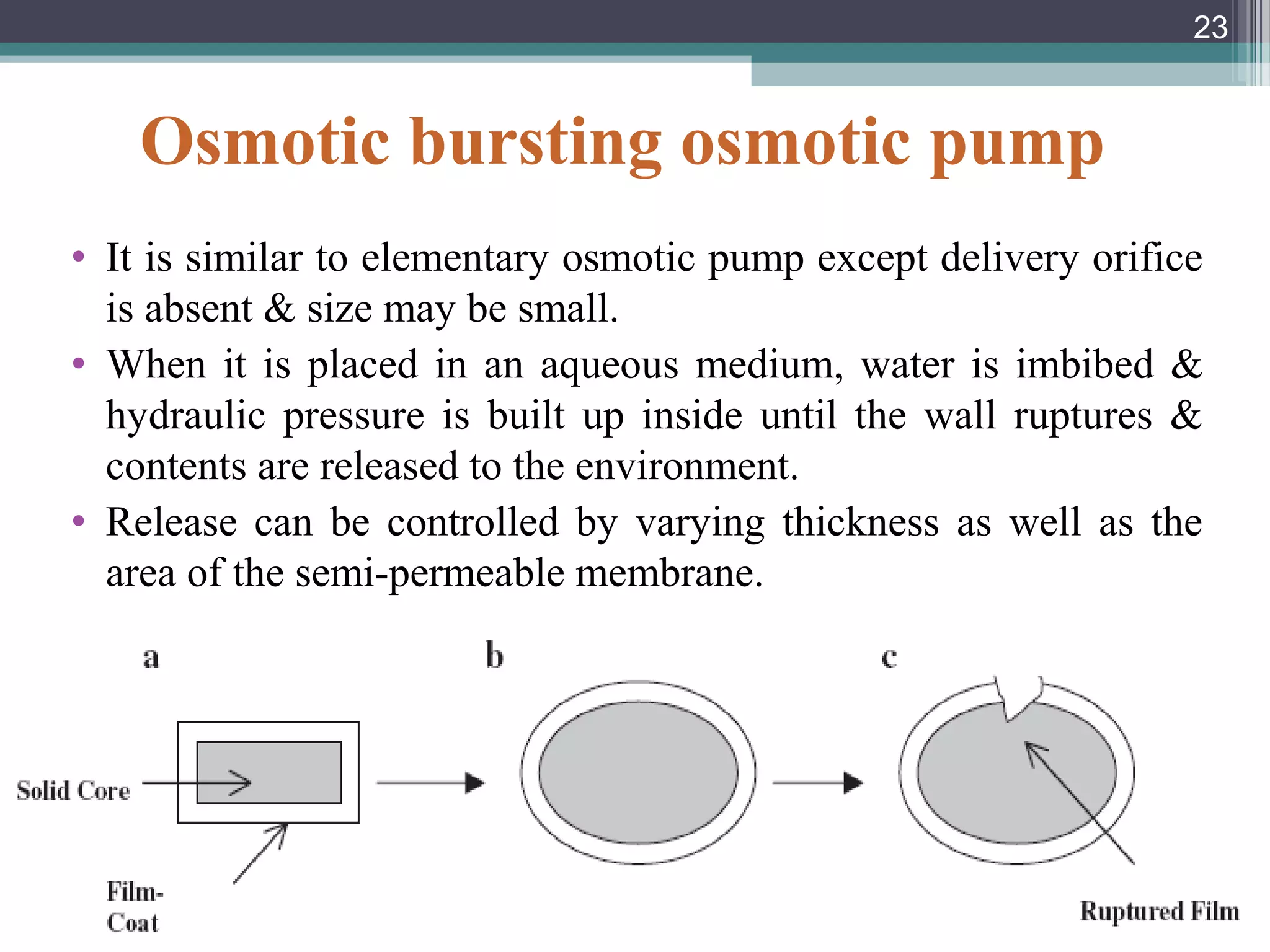
This document provides an overview of osmotically controlled drug delivery systems. It discusses the principles of osmosis that these systems utilize. Key components include a drug, osmotic agent, and semipermeable membrane. Factors that can affect the drug release rate include drug solubility, osmotic pressure, membrane characteristics, and orifice size. Various types of osmotic pumps are classified and described, including oral and implantable versions. Commercial applications and evaluation methods are also mentioned.













![Very rapid gelling and nearly complete hydration of OCAS delivery system in the upper GI tract ensures drug release
throughout the entire GI tract,
including the colon where water is poorly available. Reprinted from European Urology Supplements, 4(2), Michel MC,
Korstanje C, KrauwinkelW, Kuipers M,
The pharmacokinetic profile of tamsulosin oral controlled absorption system (OCAS1), pp 15–24, 2005, with permission
from European Association of
Urology [8].
37](https://image.slidesharecdn.com/nileshseminar-160412060758/75/Osmatically-Controlled-Drug-Delivery-System-37-2048.jpg)