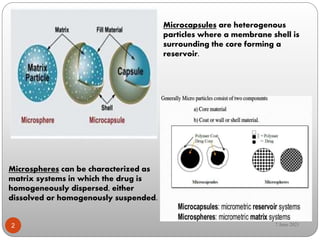
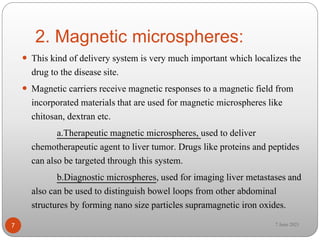
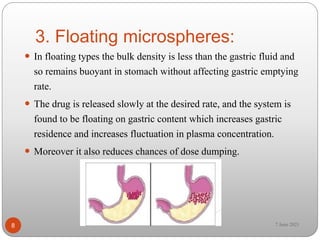
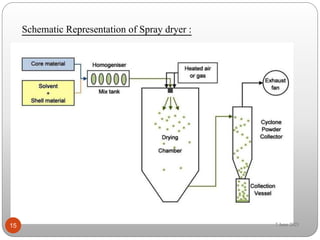
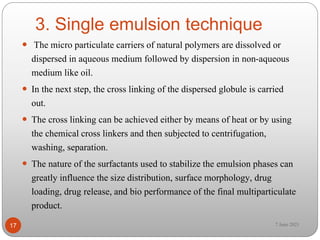
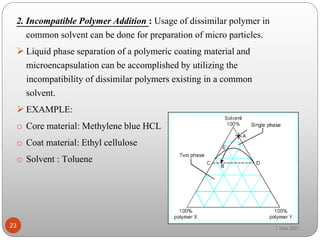
This document discusses microspheres and microcapsules. It defines microspheres as solid spherical particles ranging from 1-1000μm that can be matrix systems with drug dispersed throughout or reservoir systems with drug enclosed. The document describes various types of microspheres including bioadhesive, magnetic, floating, and radioactive. It also discusses common polymers used and various preparation techniques such as spray drying, solvent evaporation, and polymerization. Finally, the document outlines methods for evaluating properties of microspheres like particle size, drug loading, and in vitro drug release.