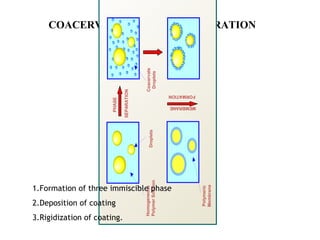
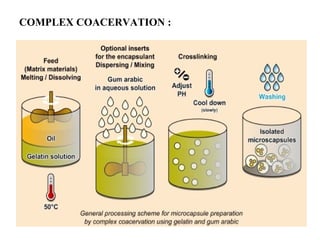
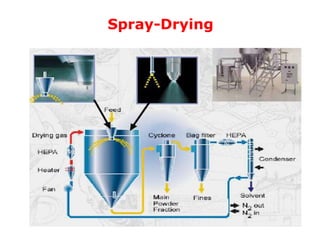
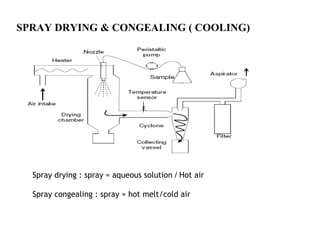
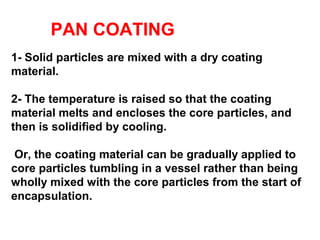
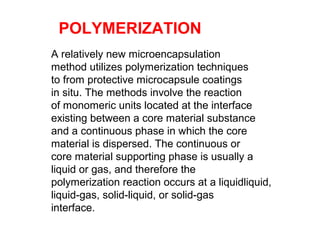
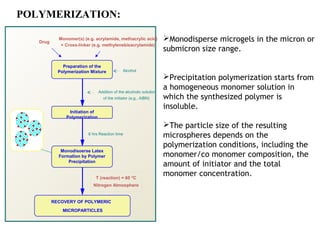
Microencapsulation is a process where core materials are surrounded by a continuous film of polymeric material to form microparticles or microcapsules between 3-800μm in size. There are various techniques to microencapsulate such as spray drying, pan coating, and polymerization. Microencapsulation can increase bioavailability, alter drug release, and provide targeted drug delivery. Evaluation of the microcapsules involves measuring yield percentage, particle size, drug content, encapsulation efficiency, and in vitro drug release.










![EVALUATION OF MICROCAPSULES
Percentage Yield
The total amount of microcapsules obtained was weighed and
the percentage yield calculated taking into consideration the
weight of the drug and polymer [7].
Percentage yield = Amount of microcapsule obtained /
Theoretical Amount×100
Scanning electron microscopy
Scanning electron photomicrographs of drug loaded ethyl
cellulose microcapsules were taken. A small amount of
microcapsules was spread on gold stub and was placed in the
scanning electron microscopy (SEM) chamber.
The SEM photomicrographs was taken at the
acceleration voltage of 20 KV.](https://image.slidesharecdn.com/microencapsulation2-121016104845-phpapp01/85/Microencapsulation-2-27-320.jpg)
![Particle size analysis
For size distribution analysis, different sizes in a
batch were separated by sieving by using a set of
standard sieves. The amounts retained on
different sieves were weighed [5].
Encapsulation efficiency [8]
Encapsulation efficiency was calculated using
the formula:
Encapsulation efficiency = Actual Drug Content /
Theoretical Drug Content ×100](https://image.slidesharecdn.com/microencapsulation2-121016104845-phpapp01/85/Microencapsulation-2-28-320.jpg)
![Estimation of Drug Content
Cefotaxime sodium drug content in the microcapsules was
calculated by UV spectrophotometric (Elico SL159 Mumbai
India) method.
The method was validated for linearity, accuracy and
precision. A sample of microcapsules equivalent to 100 mg
was dissolved in 25 ml ethanol and the volume was
adjusted upto 100 ml using phosphate buffer of pH 7.4. The
solution was filtered through Whatman filter paper. Then the
filtrate was assayed for drug content by measuring the
absorbance at 254 nm after suitable dilution [9].](https://image.slidesharecdn.com/microencapsulation2-121016104845-phpapp01/85/Microencapsulation-2-29-320.jpg)



