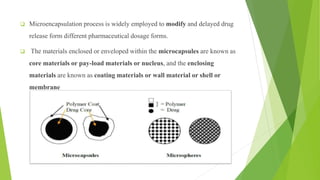
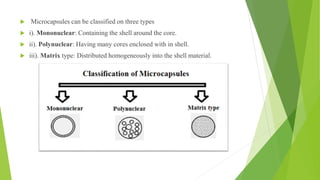
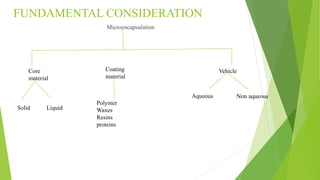
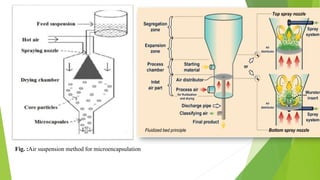
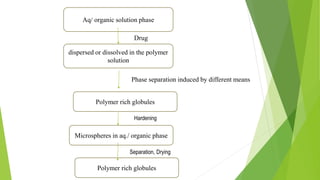
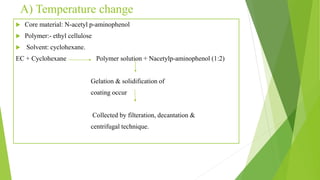
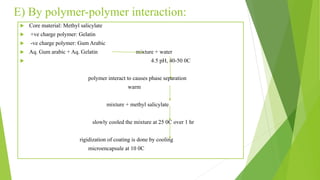
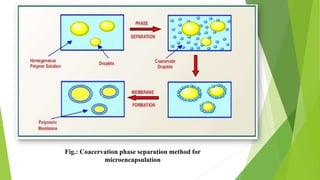
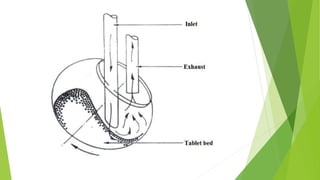
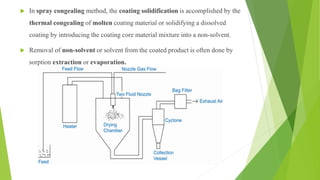
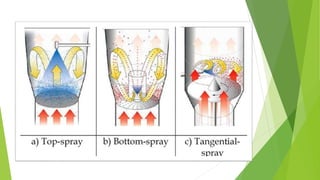
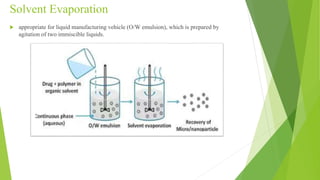
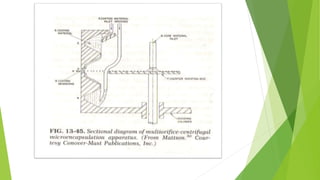
This document provides information about microencapsulation including definitions, advantages, disadvantages, types of microparticles, and methods of encapsulation. Microencapsulation is defined as enclosing solids, liquids, or gases within a polymeric coating to form microparticles 1-1000 μm in size. Common methods include spray drying, solvent evaporation, pan coating, and fluidized bed coating. Microencapsulation can provide environmental protection, control release rates, and mask unpleasant tastes. It has applications in fields like drug delivery, agriculture, and food technology.