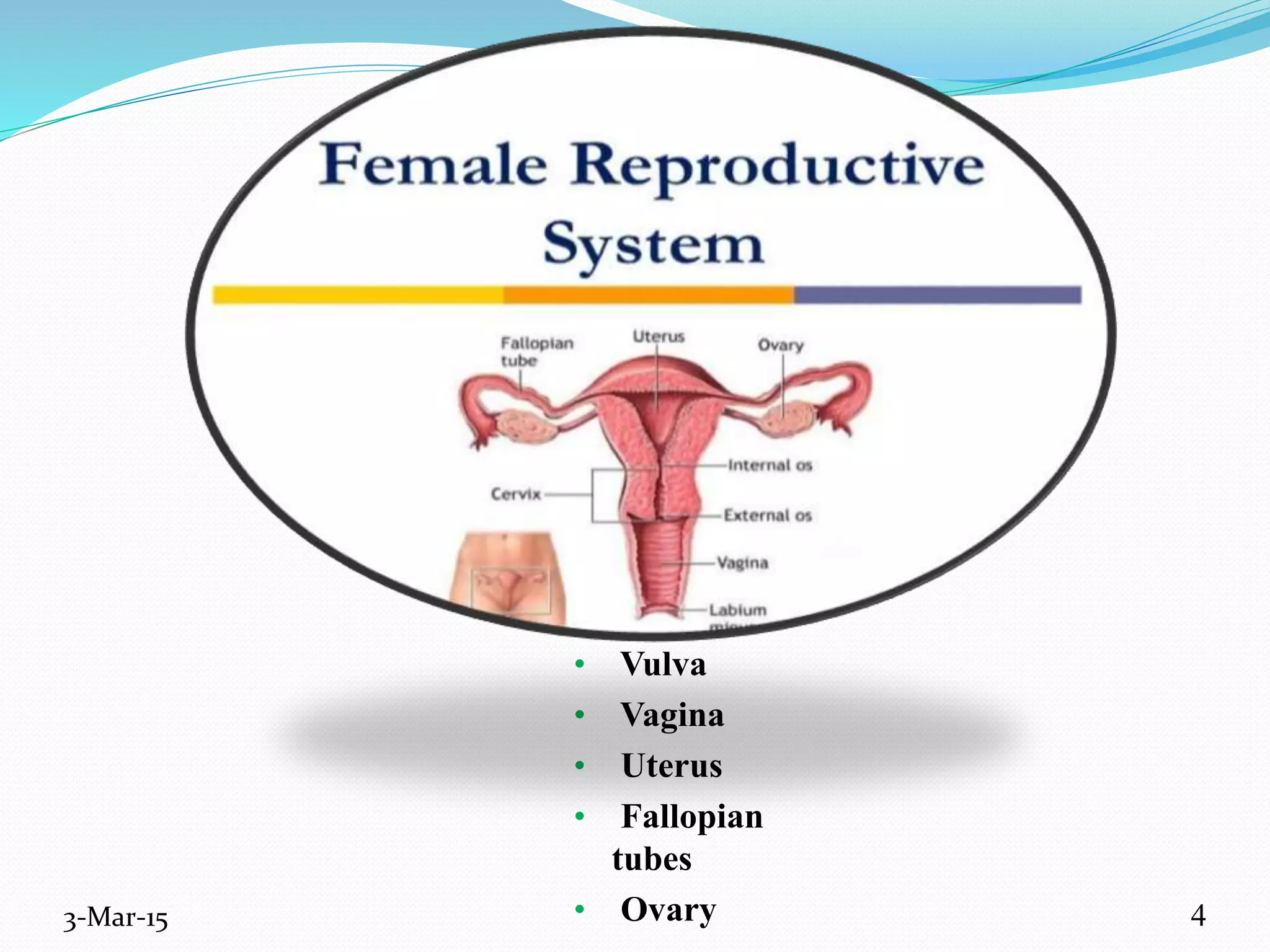
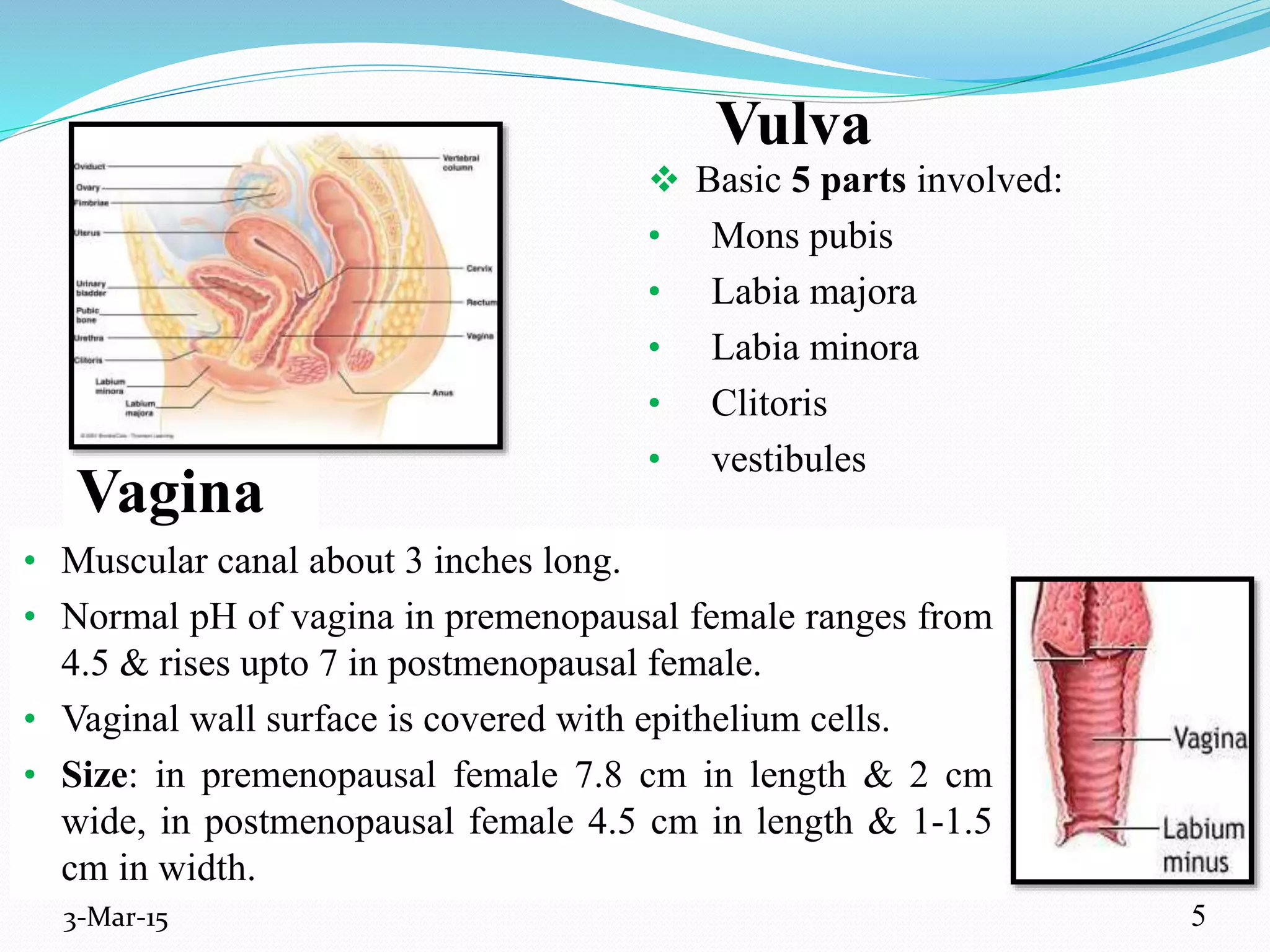
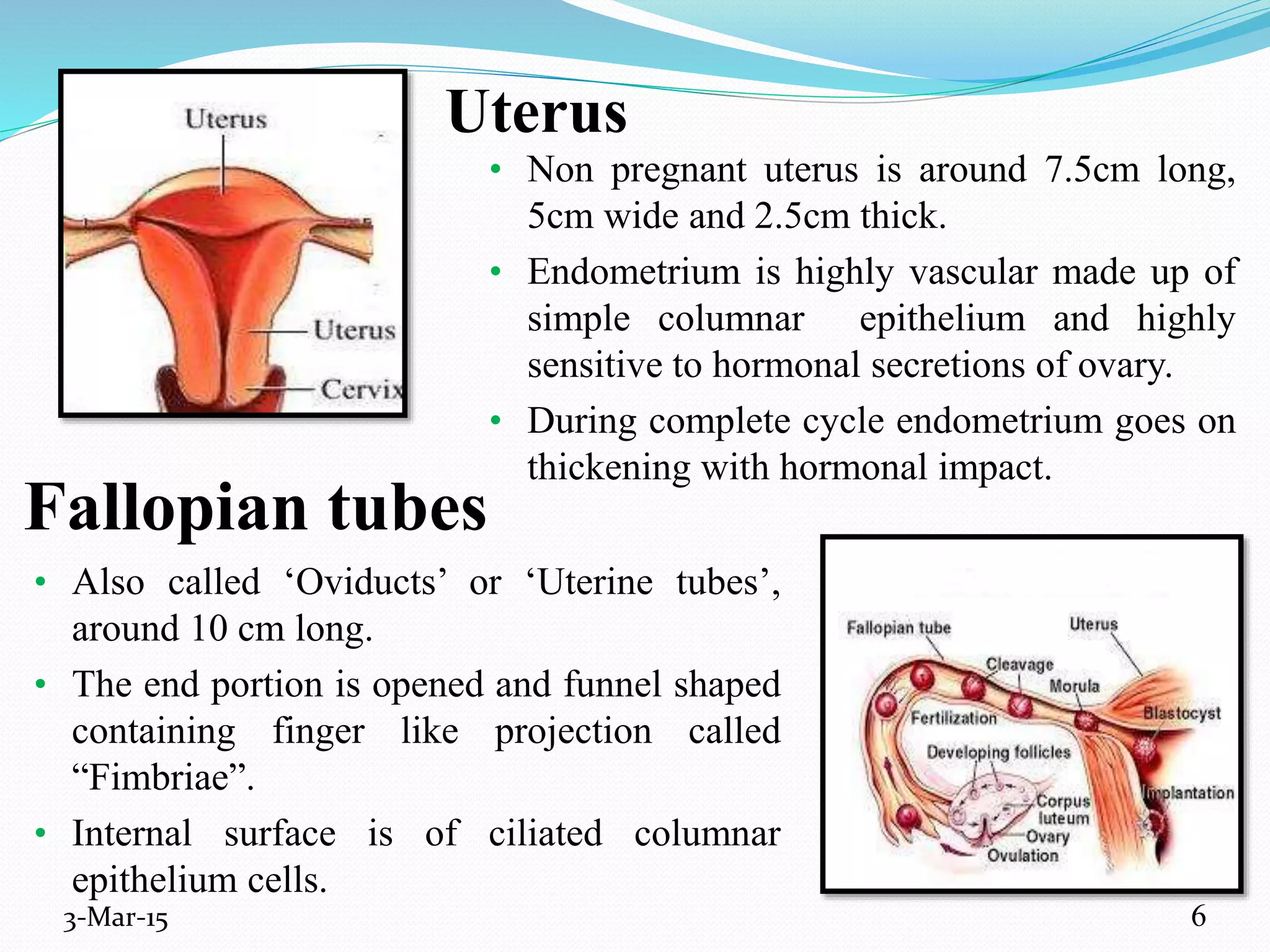
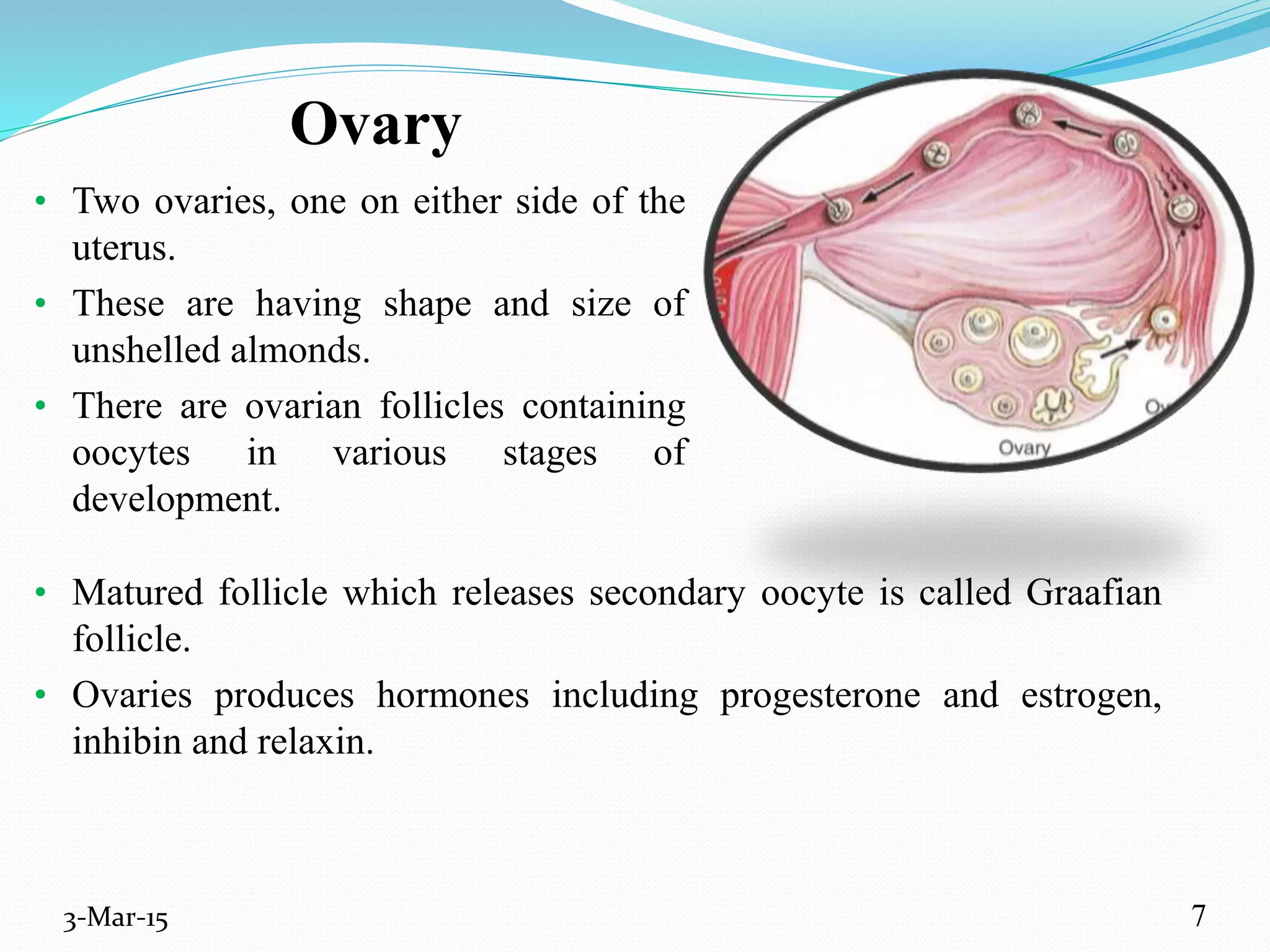
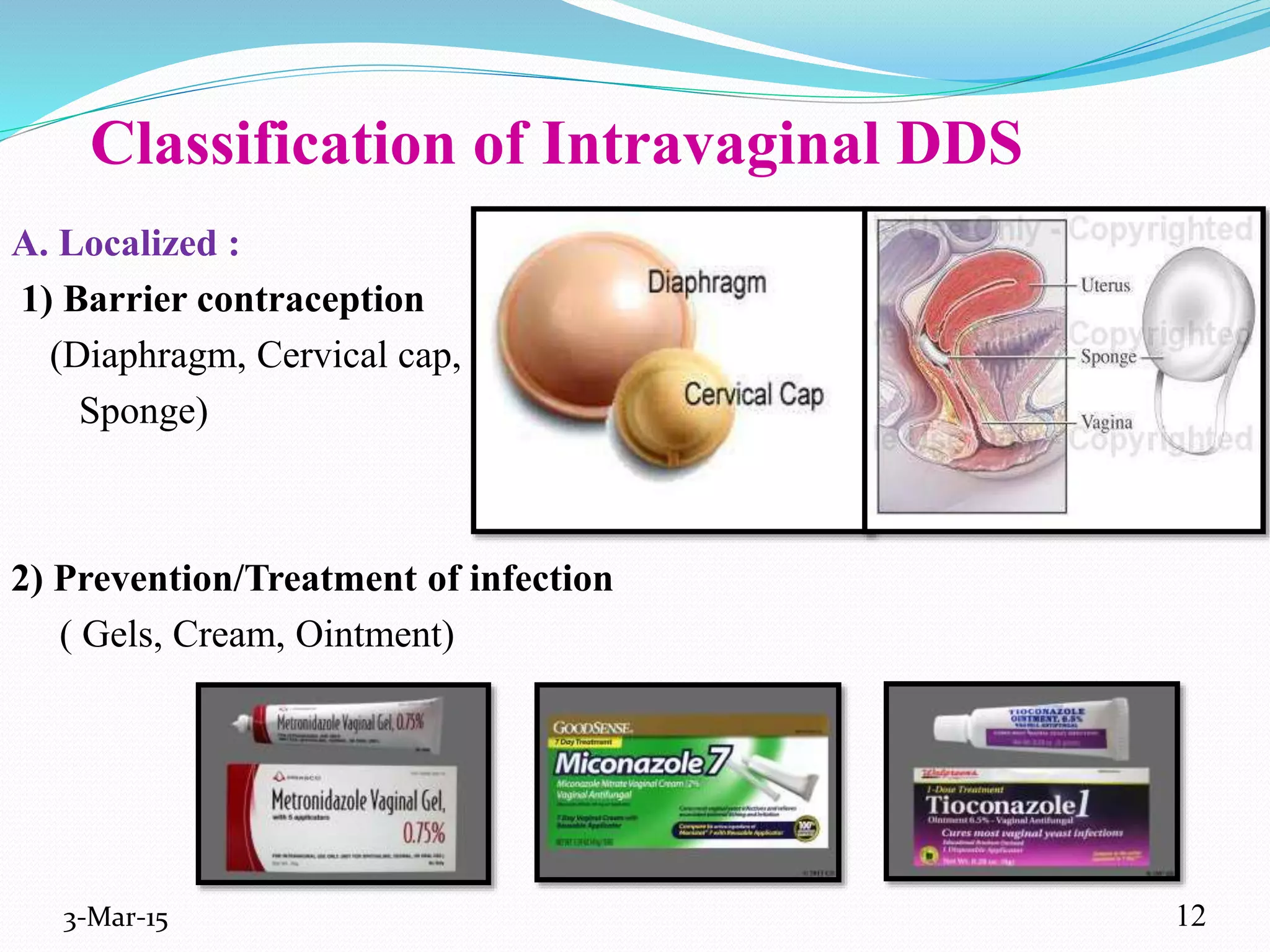
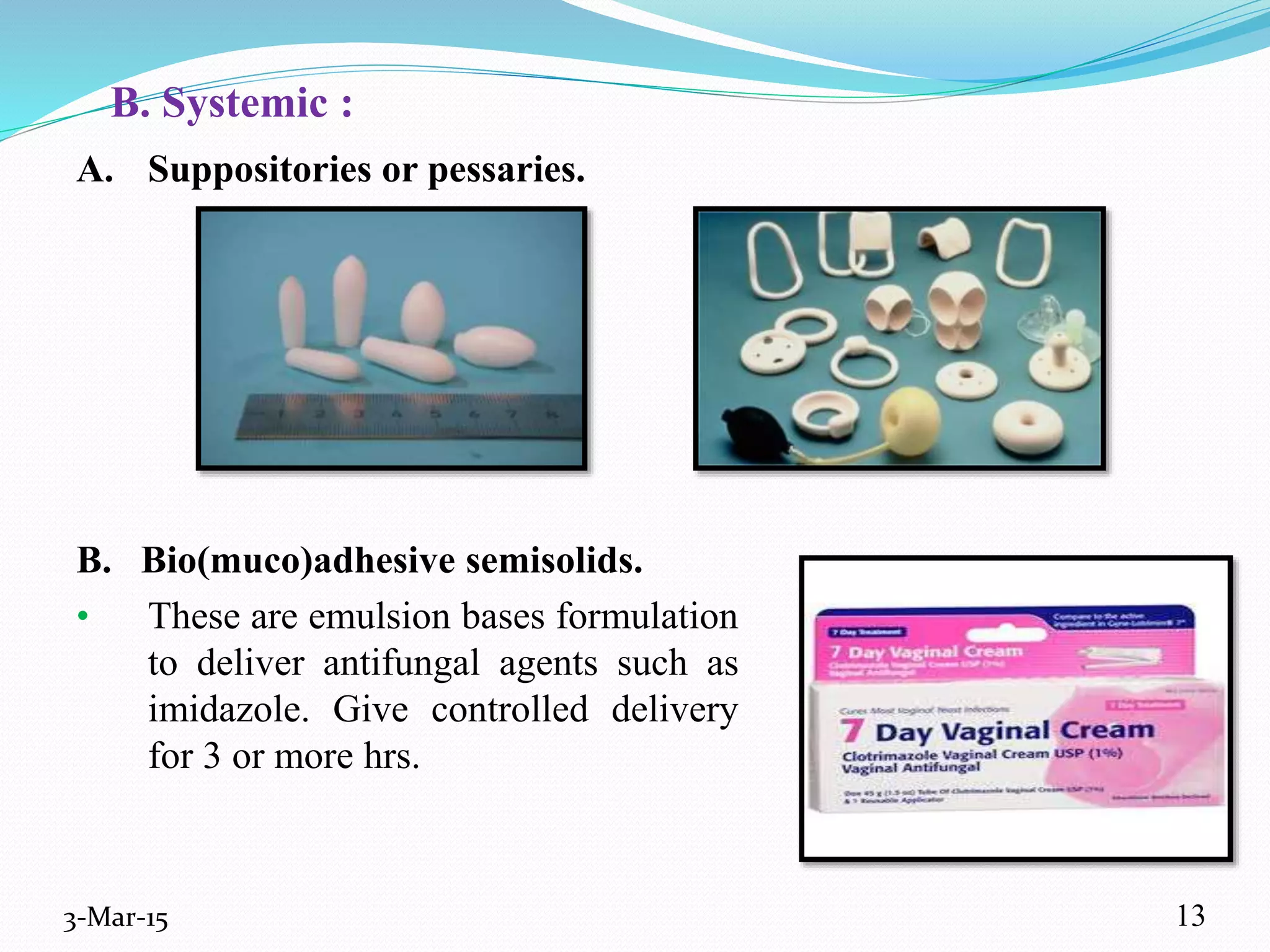
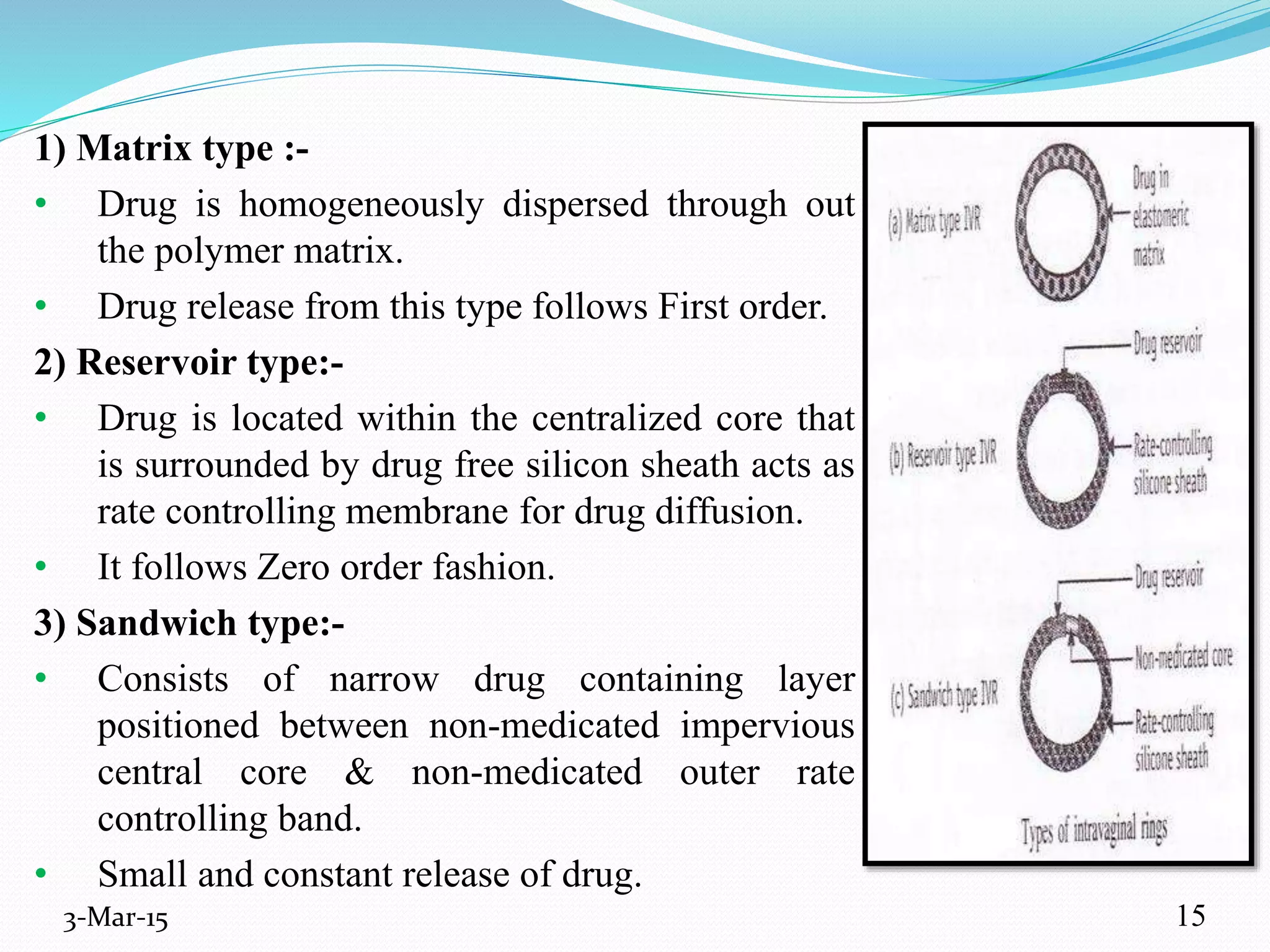
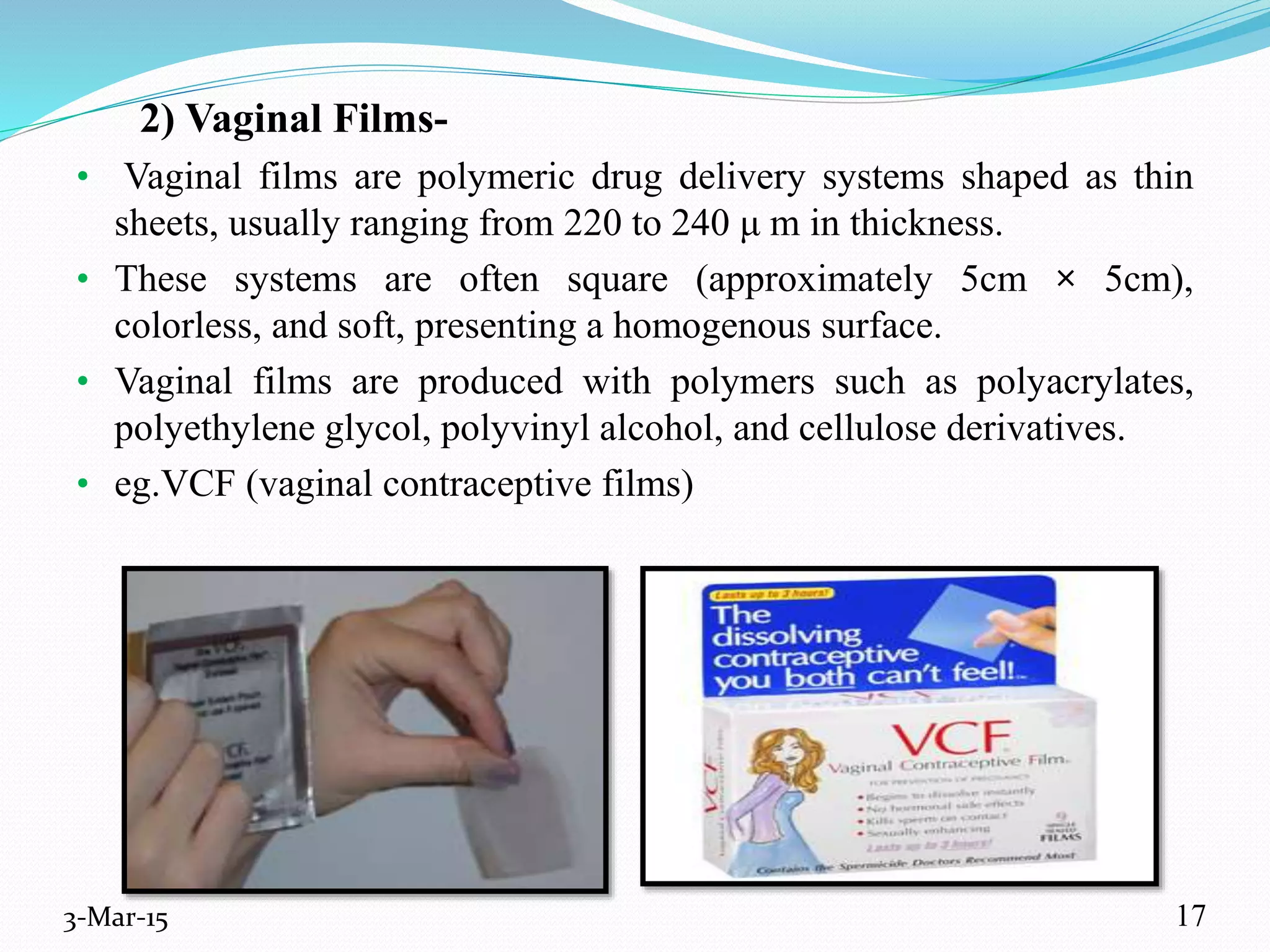
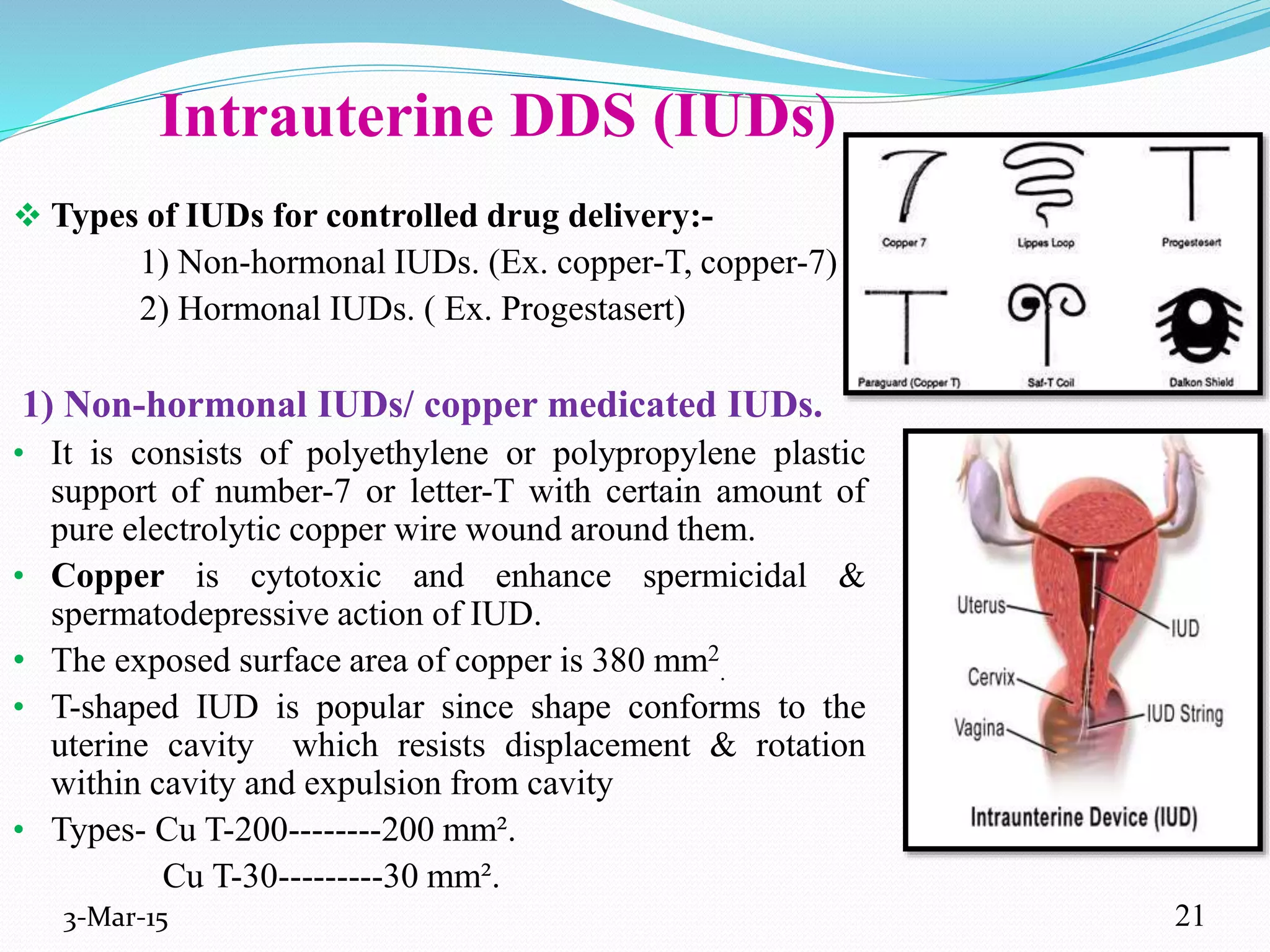
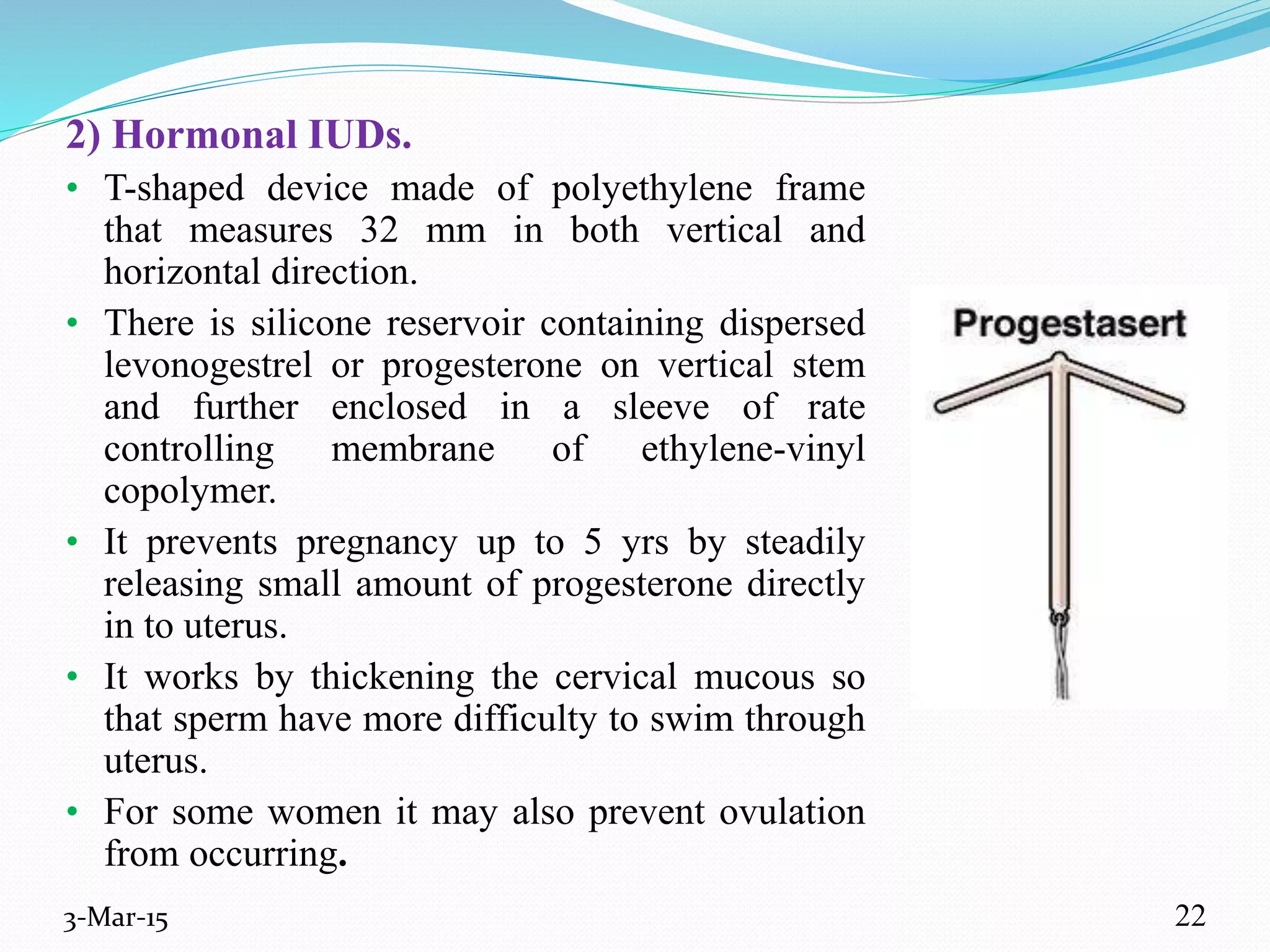
This document discusses intrauterine and intravaginal drug delivery systems. It begins with an introduction and overview of anatomy and physiology of the female reproductive system. It then describes various types of intravaginal drug delivery systems including suppositories, bioadhesive semisolids, elastomeric rings, and solid polymeric carriers. Factors affecting vaginal drug absorption are also discussed. The document concludes by describing intrauterine drug delivery systems including non-hormonal and hormonal IUDs, and discussing advantages and disadvantages of both intravaginal and intrauterine systems.