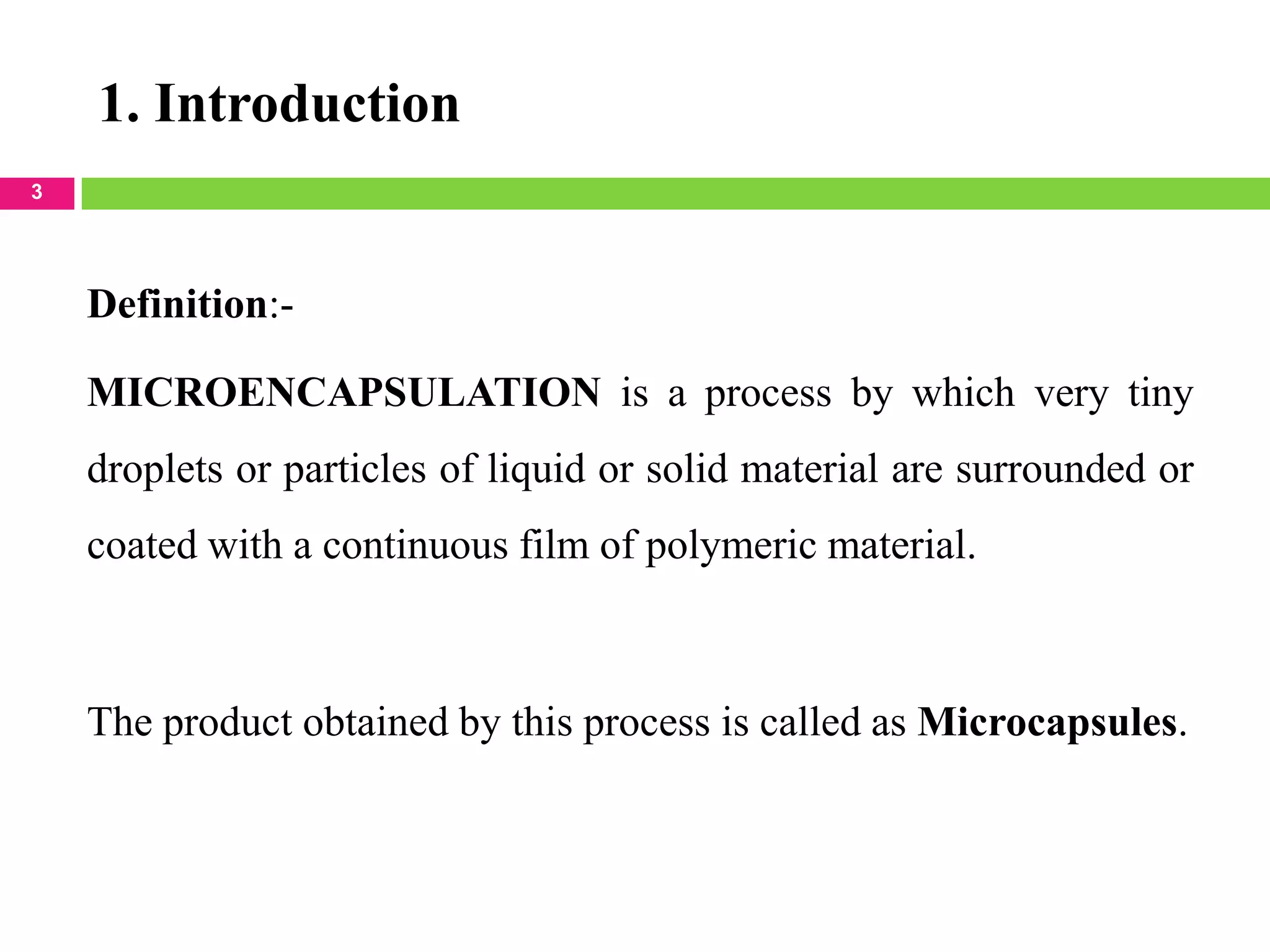
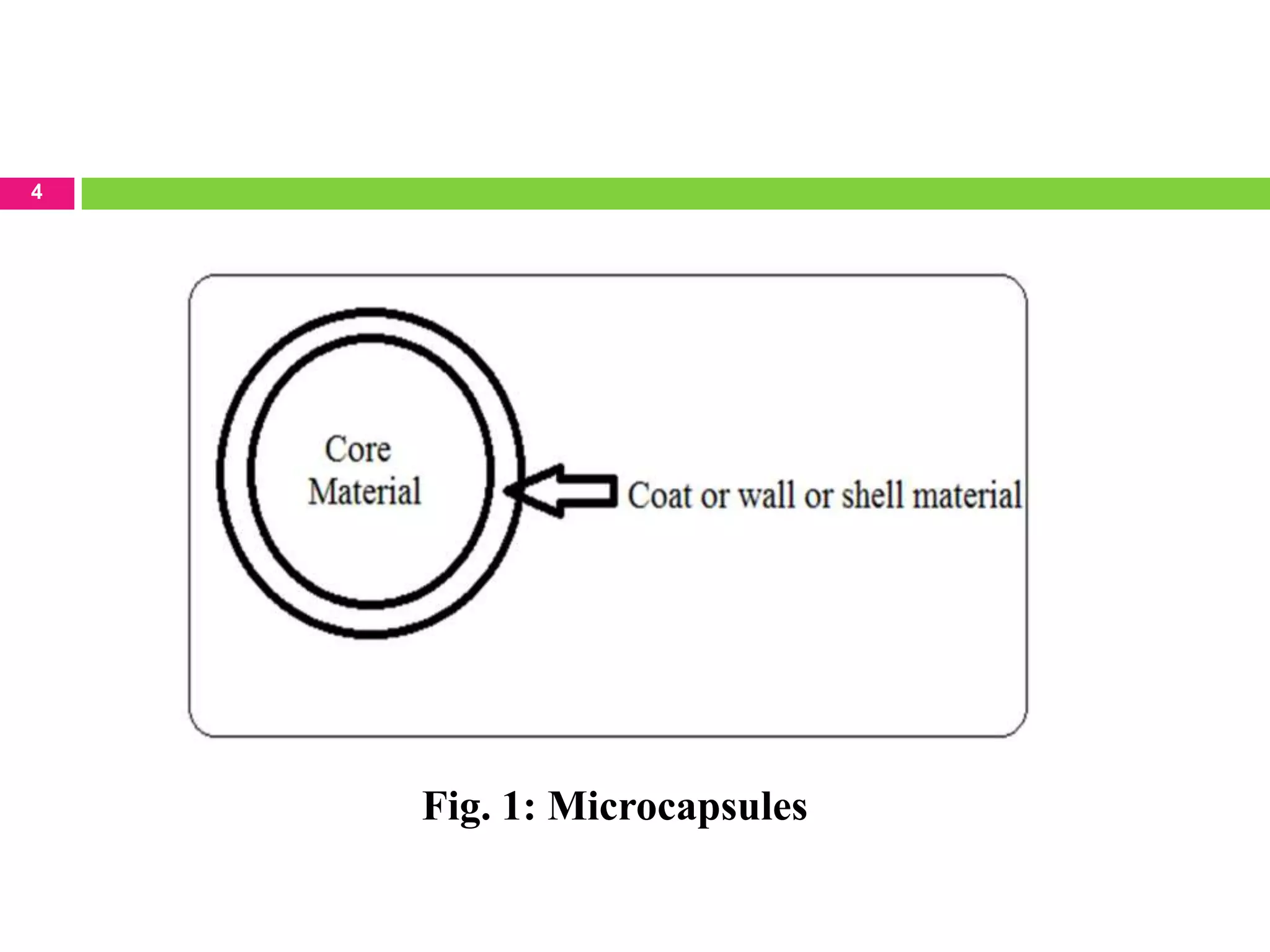
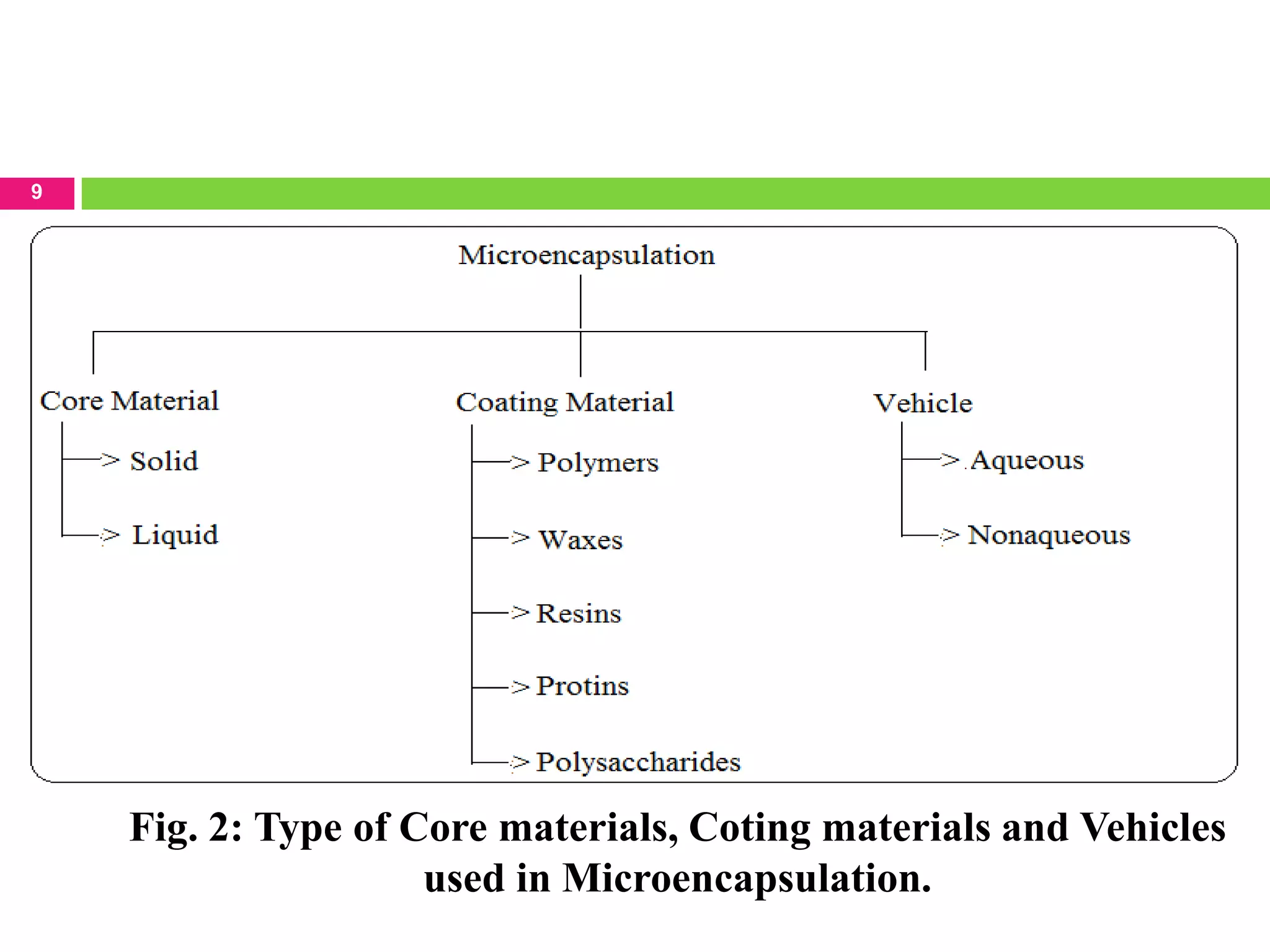
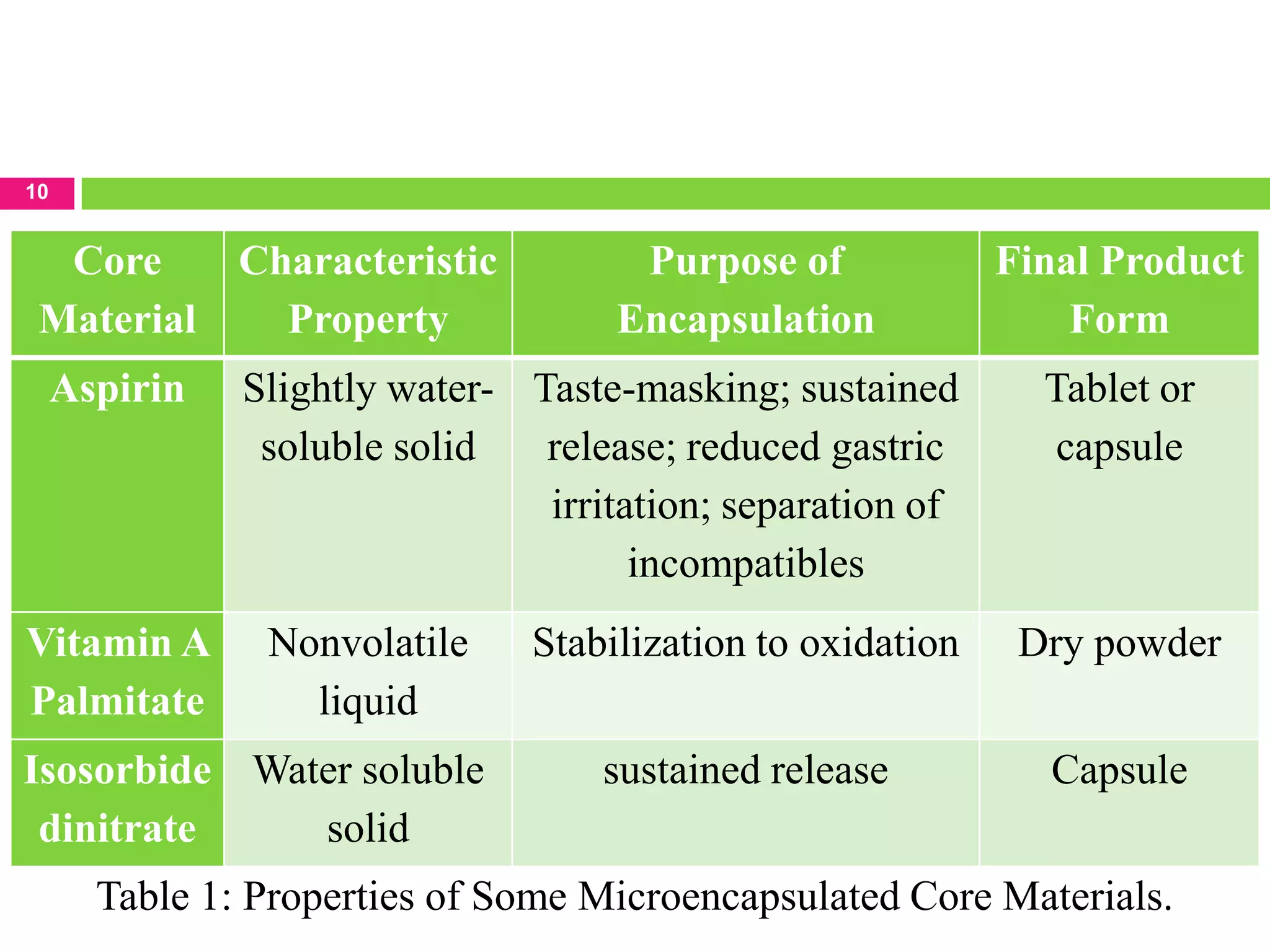
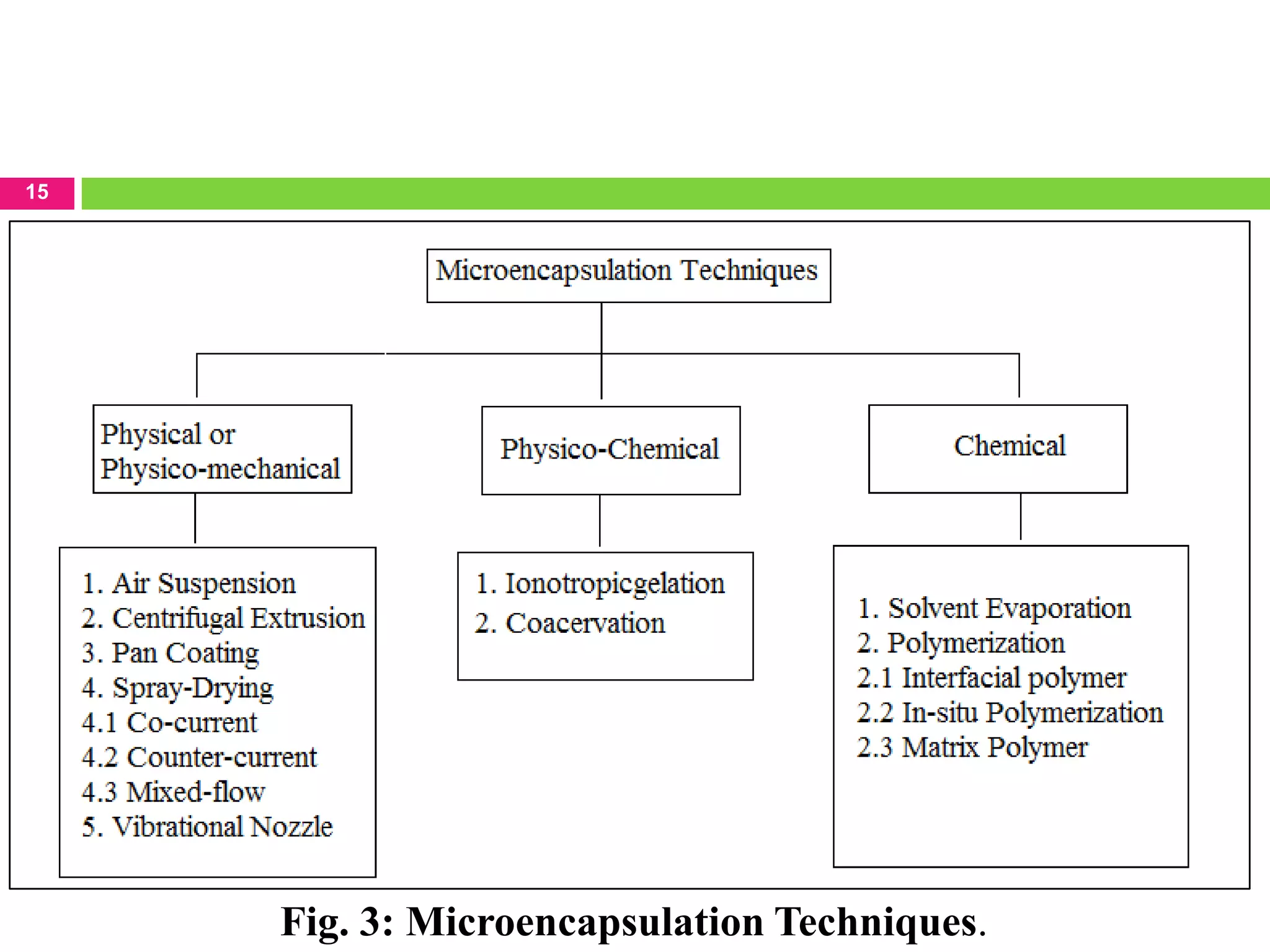
The seminar presented by Sagar B. Thoke focuses on microencapsulation techniques, emphasizing the definition, reasons for encapsulation, manufacturing techniques, and various applications. Microencapsulation involves enclosing core materials with polymeric coatings to protect them and control their release, which has significant implications in pharmaceuticals, agriculture, and food industries. Techniques discussed include air-suspension coating, centrifugal extrusion, spray-drying, and various physico-chemical methods, highlighting the process's complexity and industrial applicability.










![I] Physical or Physico-mechanical methods
16
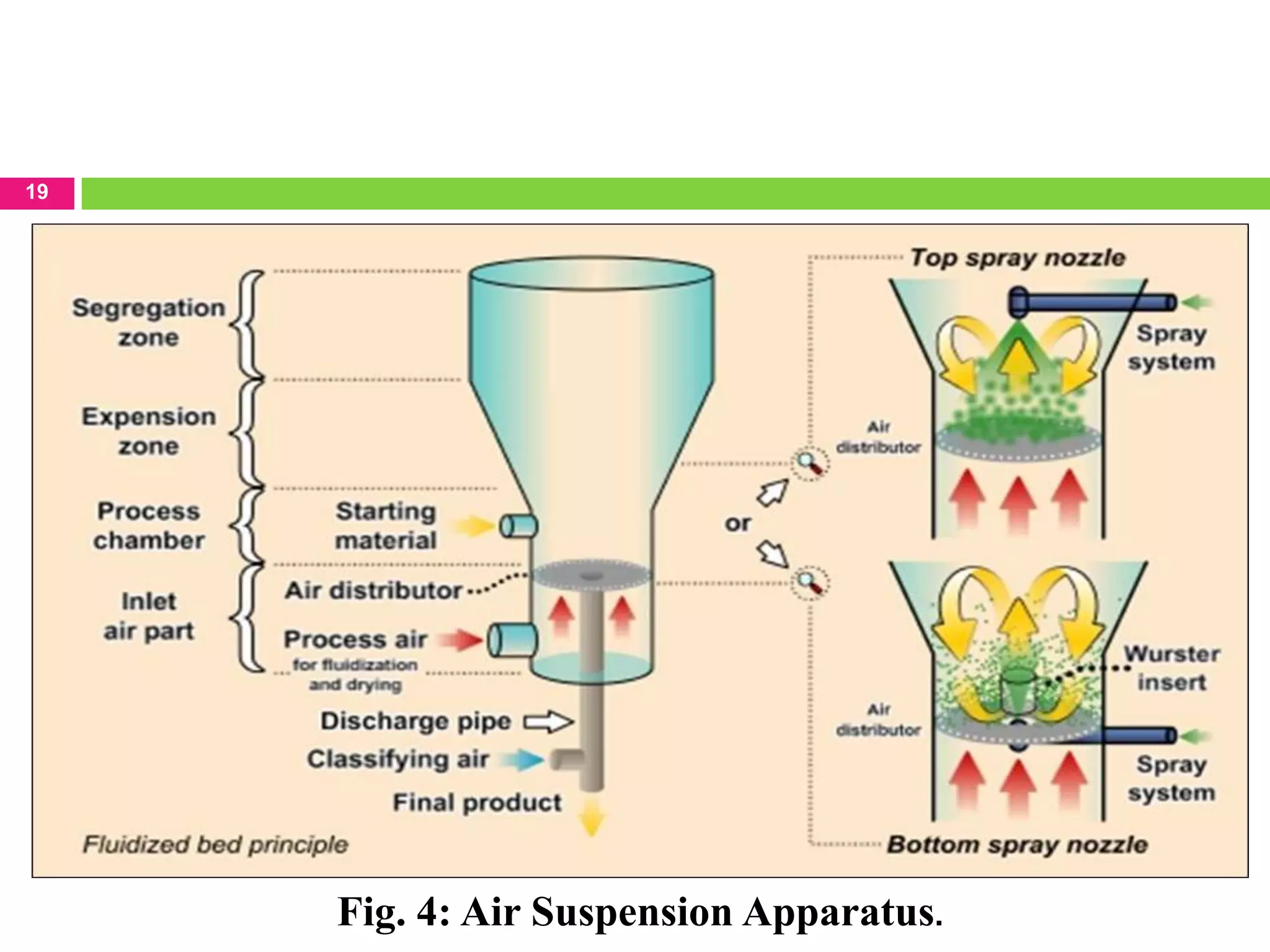
1. Air-suspension coating
Inventions of Professor Dale E. Wurster
Basically the wurster process consists of the dispersing of solid,
particulate core materials in a supporting air stream and the
spray-coating of the air suspended particles.
Equipment ranging in capacities from one pound to 990 pounds.
Micron or submicron particles can be effectively encapsulated
by air suspension techniques.](https://image.slidesharecdn.com/sagarthoke-120411004844-phpapp02/75/MICROENCAPSULATION-TECHNIQUES-AND-APPLICATION-16-2048.jpg)








![II] Physico-chemical methods
29
1. Ionotropic gelation
Chemical reaction between sodium alginate and calcium
chloride or other Counter ion solution such as barium chloride.
Verapamil hydrochloride causes gastric irritation on sudden
release. It is usually administered as conventional tablets
containing 40-120 mg, 3 times a day. Due to its ready solubility
in water and shorter half-life.
Microparticulate system of verapamil hydrochloride for
prolonged release delivery system.](https://image.slidesharecdn.com/sagarthoke-120411004844-phpapp02/75/MICROENCAPSULATION-TECHNIQUES-AND-APPLICATION-29-2048.jpg)

![III] Chemical process
32
1. Solvent Evaporation
In the case in which the core material is dispersed in the
polymer solution, polymer shrinks around the core. In the case
in which core material is dissolved in the coating polymer
solution, a matrix - type microcapsule is formed.
The core materials may be either
water - soluble or
water - insoluble materials.
A variety of film - forming polymers can be used as coatings.](https://image.slidesharecdn.com/sagarthoke-120411004844-phpapp02/75/MICROENCAPSULATION-TECHNIQUES-AND-APPLICATION-32-2048.jpg)


![4] Applications of Microcapsules and Microspheres
36
1. Agricultural Applications
Reduce insect populations by disrupting their mating process.
Protects the pheromone from oxidation and light during storage
and release.](https://image.slidesharecdn.com/sagarthoke-120411004844-phpapp02/75/MICROENCAPSULATION-TECHNIQUES-AND-APPLICATION-36-2048.jpg)






![5] REFERENCES
45
Leon, L., Herbert A. L., Joseph, L. K; “ The Theory And Practice Of Industrial
Pharmacy”, 3rd edition (1990), Varghese Publishing House, Page no.- 412-428.
S.S. Bansode, a review on “Microencapsulation”, International Journal of
Pharmaceutical Sciences Review and Research, Volume 1, Issue 2, March – April 2010;
Article 008; ISSN 0976 – 044X, Page no.- 38-43.
N.K. SACHAN, a review on “Controlled Drug Delivery Through Microencapsula -
tion”, Malaysian Journal of Pharmaceutical Sciences, Volume 4, No. 1, Page no.- 65–81
(2006).
H. Umer, a review on “Microencapsulation: Process, Techniques and Applications”,
International Journal of Research in Pharmaceutical and Biomedical Sciences, Vol. 2 (2)
Apr – Jun 2011, ISSN: 2229-3701, Page no.-447-481.](https://image.slidesharecdn.com/sagarthoke-120411004844-phpapp02/75/MICROENCAPSULATION-TECHNIQUES-AND-APPLICATION-45-2048.jpg)


