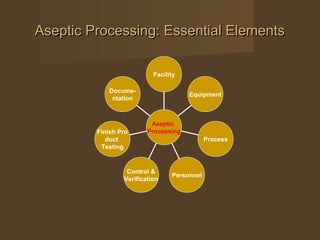
The document discusses aseptic processing, which involves bringing together sterile products, containers, and closures that have been separately sterilized and assembling them in a highly controlled environment using specialized personnel and equipment. Key elements of aseptic processing include facility design and control, equipment sterilization and material handling, the aseptic processing itself, personnel training, process verification through media fills and environmental monitoring, finished product testing, and comprehensive documentation.