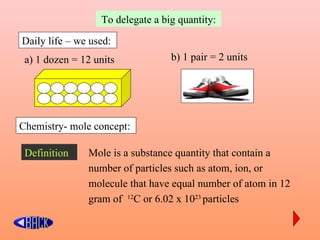
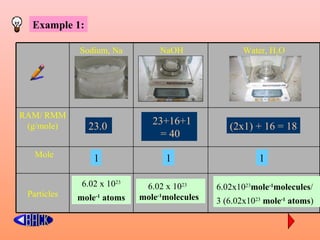
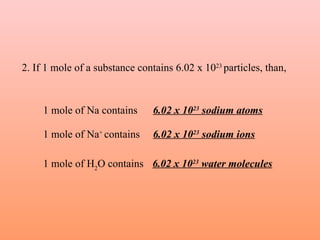
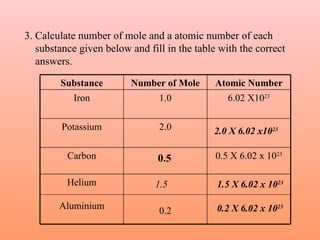
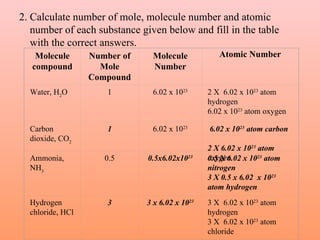
The document defines the mole concept as a quantity of particles that contains an equal number of atoms as 12 grams of carbon-12 (6.02 x 1023 particles). It also defines Avogadro's number (NA) as 6.02 x 1023 particles per mole. Examples are given of calculating the number of particles in 1 mole of various substances like sodium (Na), sodium hydroxide (NaOH), and water (H2O). The document concludes with practice problems defining the mole concept and calculating moles, molecules, and atoms for different substances.