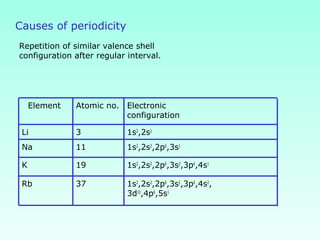
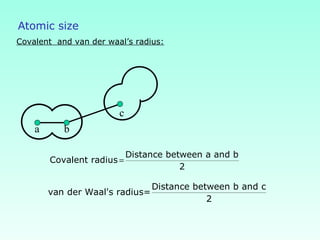
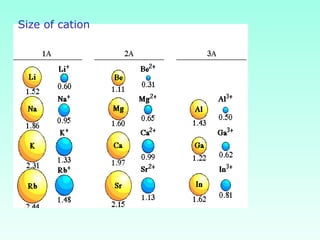
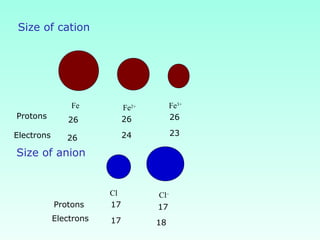
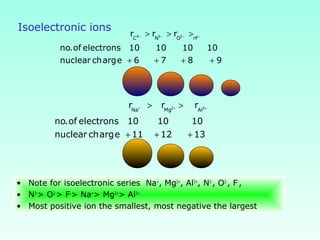
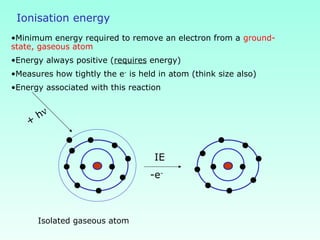
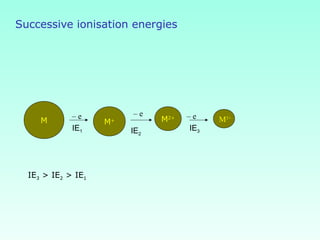
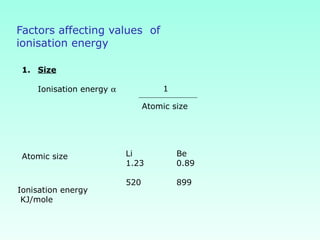
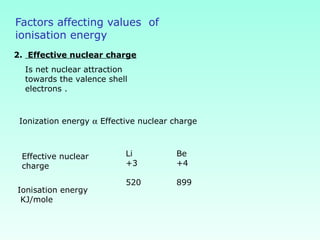
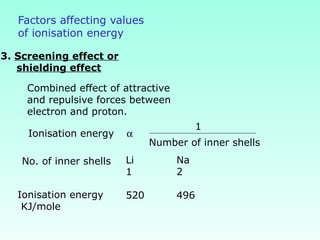
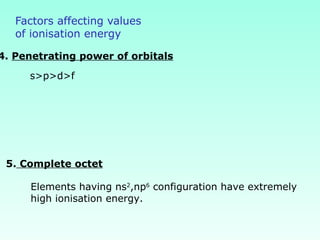
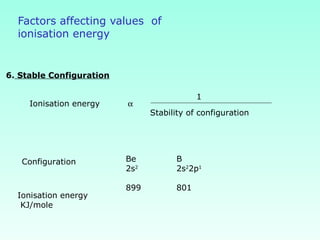
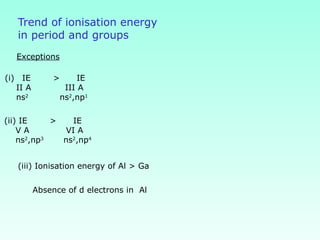
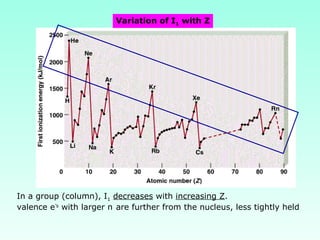
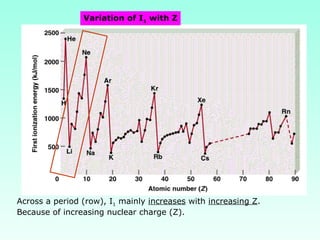
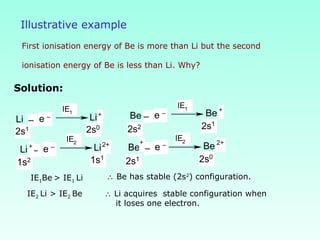
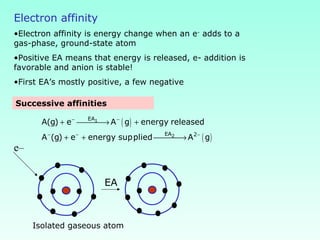
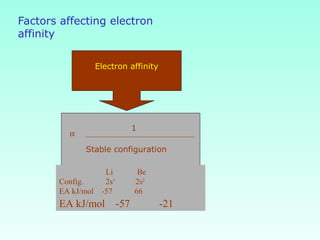
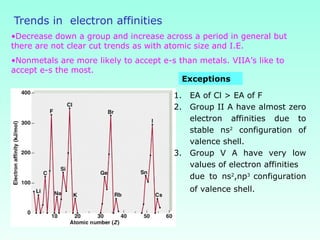
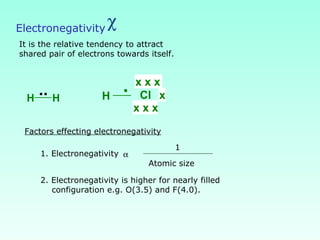
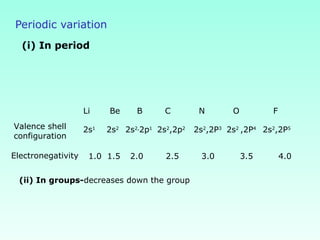
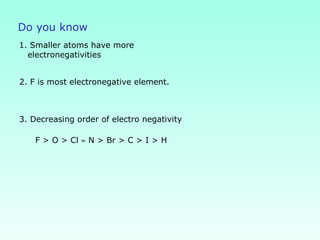
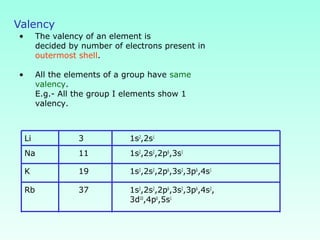
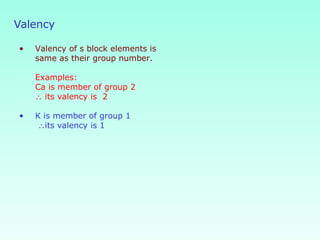
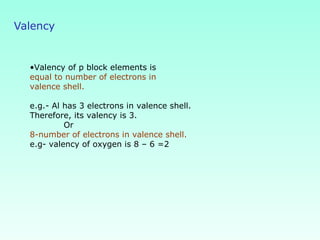
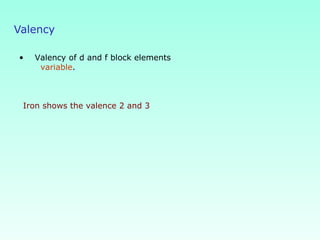
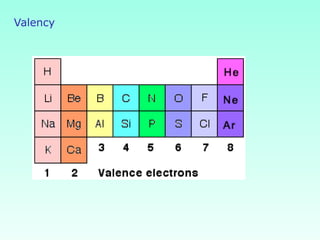
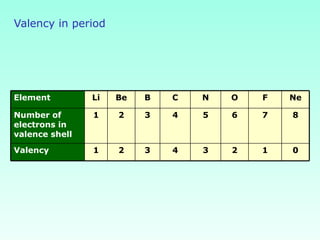
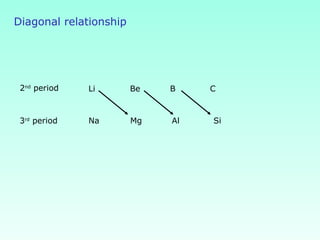
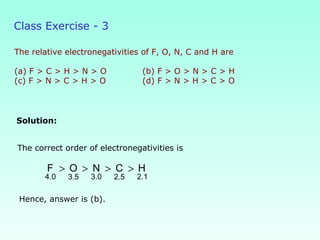
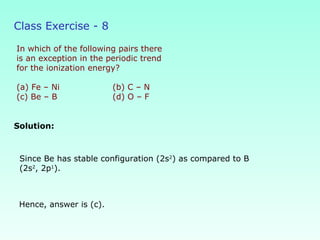
This document provides information on the classification of elements and various periodic properties like atomic size, ionization energy, electron affinity and electronegativity. It discusses the trends in these properties across periods and groups and exceptions to trends. It also explains concepts like ionic size, isoelectronic species, ionization energies, electron affinities, Pauling and Mulliken scales of electronegativity and valency. Sample problems are provided at the end to test the understanding of these concepts.