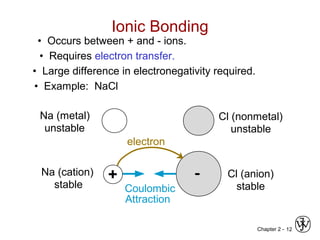
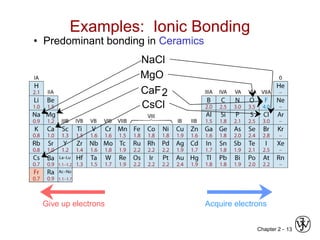
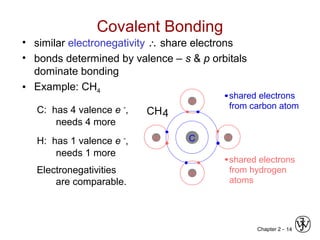
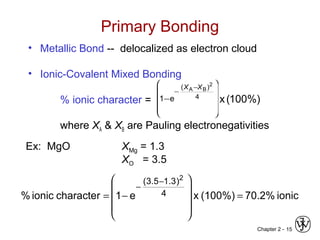
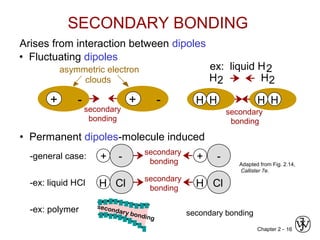
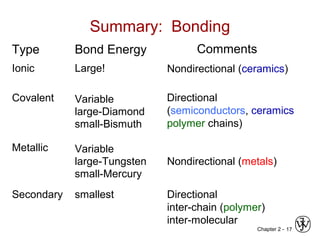
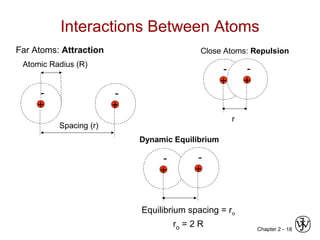
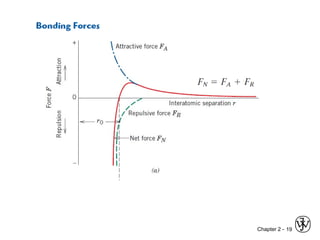
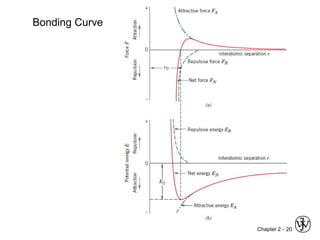
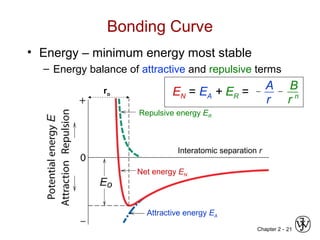
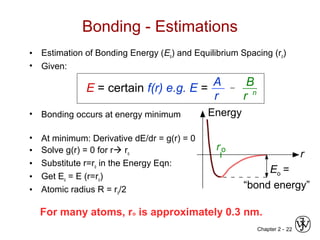
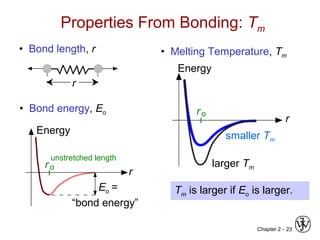
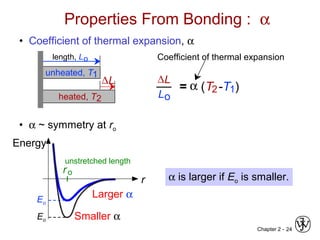
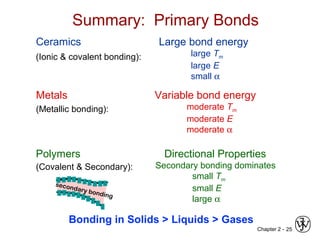
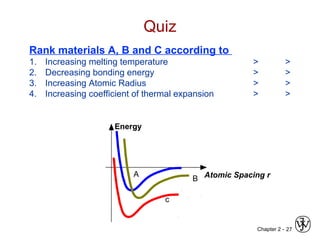
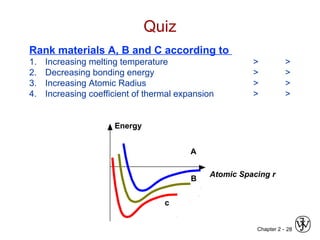
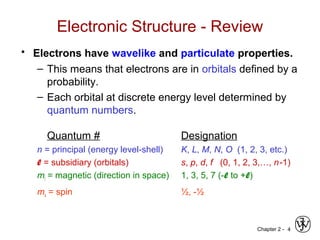
This document provides an overview of atomic bonding and properties. It discusses how the arrangement of atoms and interactions between atoms determine properties. The main types of bonds - ionic, covalent, and metallic - are introduced along with how they relate to properties like melting temperature and thermal expansion. Bonding concepts like bond energy, bond length, and the relationship between bonding strength and properties are explained through diagrams of bonding curves. The roles of factors like atomic radius, electronegativity, and bond type in determining properties are also summarized.
![Chapter 2 - 2
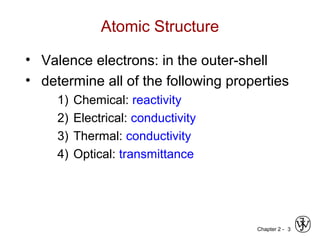
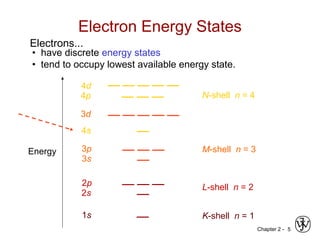
Atomic Structure (Freshman Chem.)
• atom – electrons – 9.11 x 10-31
kg
protons
neutrons
• nucleus of atom: “size of a point on a page” -! فراغ
• atomic number = No. of protons in nucleus of atom
= No. of electrons of neutral species
A [=] atomic mass unit = amu = 1/12 mass of 12
C
Atomic wt = wt of 6.023 x 1023
molecules or atoms
Unit 1 amu/atom = 1g/mol
C 12.011
H 1.008 etc.
} 1.67 x 10-27
kg](https://image.slidesharecdn.com/ch02-m-150512161306-lva1-app6891/85/Ch02-m-2-320.jpg)
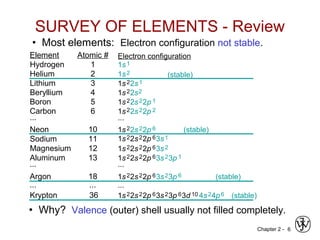
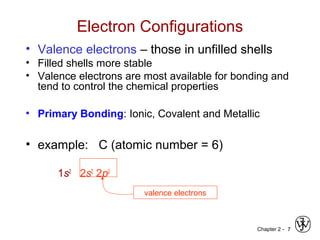
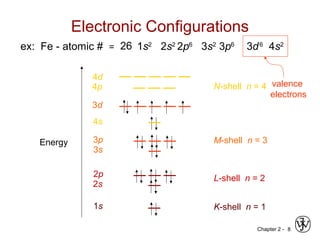
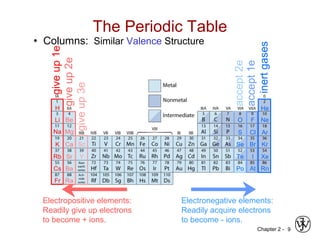
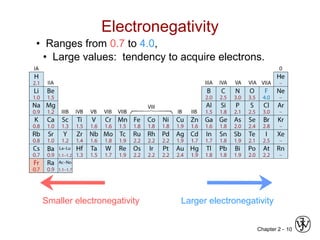
![Chapter 2 - 11
Ionic bond – metal + nonmetal
donates accepts
electrons electrons
Dissimilar electronegativities
ex: MgO Mg 1s2
2s2
2p6
3s2
O 1s2
2s2
2p4
[Ne] 3s2
Mg2+
1s2
2s2
2p6
O2-
1s2
2s2
2p6
[Ne] [Ne]
1s22s22p 6 (stable)10Neon](https://image.slidesharecdn.com/ch02-m-150512161306-lva1-app6891/85/Ch02-m-11-320.jpg)